http://www.chemistrymag.org/cji/2001/039042ne.htm |
Sep. 1,
2001 Vol.3 No.9 P.42 Copyright |
An Taicheng, Zhu Xihai, Xiong Ya, Jiang
Shaoji#
(School of Chemistry and Chemical Engineering; #State Key Laboratory of
Ultrafast Laser Spectroscopy, Zhongshan University, Guangzhou, 510275 China.)
Received on Mar.25, 2001; Supported by the Natural Science Foundation of China (29977030), Natural Science Foundation of Guangdong Province, China (990274), R. & D. Project of EPA of Guangdong province, China (1999-14) and Univ. Key laboratory of Environ. Sci. & Tech. of Henan province.
Abstract A novel
photoelectrochemical reactor, three dimension electrode-slurry photocatalytic reactor, was
designed and characterized by the current enhancement and COD removal efficiency. An
apparent photoelectrochemical synergic effect was observed for the treatment of dye
containing waste water in the reactor.
Keyword Photoelectrochemical; Photocatalytic; Three Dimension Electrode;
Reactor.
1. INTRODUCTION
The photoelectrochemical technology with external potential appears to be a research front
of photocatalytic degradation of organic pollutants. Many academic interests have been
focused on this area in recent years [1-3]. However, The main purpose of
electrochemically assisted photocatalysis is just to represent a proof-of-concept that
anodic bias on TiO2 electrode can drive away the photogenerated-electron and
-hole in different directions, and reduce their recombination. Therefore, in the most of
electrochemically assisted photocatalytic experiments, the applied anodic bias is almost
controlled to be lower than the oxidation potential of the investigated organic pollutant
so that no direct electrochemical oxidation interferes with the photocatalysis [1-4].
To date, the electro-assisted photocatalysis at higher voltage has scarcely been
investigated.
It is worthy of notice that recently the interest in the application of
electrochemical oxidation to the wastewater treatment has been increased [5-6].
In particular, the electrochemical technologies based on three dimension electrodes for
wastewater treatment have attracted much more attention [6-8] because the
electrodes are characterized with large specific surface areas and high performance in
comparison to conventional two-dimensional electrode [5-6].
Recently, we have started to investigate a novel hybrid
photoelectrochemical technology for organic wastewater treatment based on three dimension
electrode and slurry photocatalysis of semiconductor. The aim of the present paper is to
report a new-designed reactor, three dimension electrode-slurry photocatalytic reactor,
and its characterization of the current enhancement and COD removal efficiency in a wider
ranger of voltage.
2. EXPERIMENTAL
2.1 Material
The photocatalyst used was TiO2 (Degussa P25). The dye, reactive brilliant red
X-3B prepared to 1.0 mmol·L-1 (COD: 265.9
ppm). The granulated activated carbon (GAC) was used as the filler of three dimension
electrode. It has a surface area of 870.0 m2·g-1,
an average particle size of 3.5×5.1 mm and ash content of 8.1%.
A CHI650A electrochemical system, which is computerized, was used as a potentiostat.
2.2 Photoelectrochemical Reactor
The experimental set-up is presented in Fig.1. It is an open double-layered reactor (28.0
cm×6.0 cm×10.0 cm) made of
polytetrafluoroethylene plate. The stainless steel anode and cathode (main electrode) were
equipped across the reactor. 200.0g GAC used as particle electrodes were packed between
the two main electrodes situated 26.6 cm apart from each other and the suspension of RBRX
containing 1.0g titanium dioxide was added into the reactor. When the compressed air was
sparged into the reactor by a micropore plate from the bottom of reactor cell, it appeared
two layers. The upper one was mainly the suspension of TiO2 with a height of
about1.0 cm, and the lower one was mainly the GAC particle electrode with a packed height
of about 1.0 cm. A 500 W high--pressure mercury lamp was located 12.0 cm above the reactor
as an illuminant.
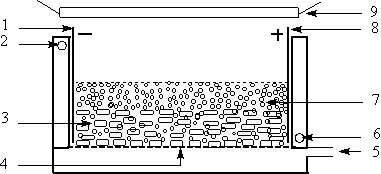
Fig.1 Schematic
diagram of three dimension electrode--photocatalytic reactor
1-Cathode; 2--Outlet of recycled water; 3--GAC
layer; 4--Micropore plate; 5--Inlet of compressed air; 6--Inlet of recycled water;
7--Titanium dioxide layer; 8--Anode; 9--UV illuminant.
3. RESULTS AND
DISCUSSION
3.1 Dependence of Voltage on Current
The photocurrent of the cell is one of major parameters characterizing photoreactor. Thus,
it was extensively investigated in the electrochemically assisted photocatalytic process.
Kraeutler et al. [9] first recognized that semiconductor particulate in the
slurry cell might have similar performance with film electrode in the photoelectrochemical
cell. They and other researchers have studied in detail the photocurrent-voltage curves
for slurry electrode, and found that the photocurrent is very small and lower than 0.2mA [10-11],
although the slurry electrode has the great advantage of good mass-transfer. However, the
photocurrent of the thin film reactor is much higher than that of the slurry reactor and
no less than 25mA [12-15].
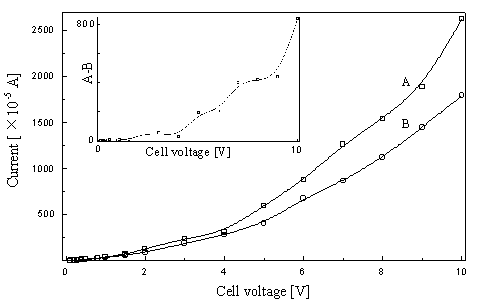
Fig.2 Dependence of voltage on
current
A--Current at 10 second in the electrochemical process; B--Current at 10 second in the
photoelctrochemical process;
In our
experiment, a high photocurrent, about 12.6mA, was observed in the three dimension
electrode-slurry photocatalytic reactor, and it is about 60 folds more than that of the
reported slurry reactor [11], although it is lower than that of the above
mentioned thin film reactor. This can be considered as an enhancement effect of three
dimension electrode to slurry photocatalytic reactor. The enhancement effect can be
attributed to that the three dimensional electrode with expanded specific area has a large
collecting area of photo-generated electron. It is expected that this kind of reactor may
have high treatment efficiency for pollutants because the magnitude of the photocurrent of
the cell is an indicator of the rate of oxidation at the anode [15].
The change of the current
with cell voltage in the reactor was also measured and shown in Fig.2. Both current of
photoelectrochemical and electrochemical process increased significantly with the increase
of applied voltage. Furthermore, the current difference of the photoelectrochemical and
the electrochemical process also increased as the increase of the voltage shown in the
inset of Fig. 2. This result indicated that the higher the applied voltage, the stronger
the ability of capturing photogenerated electron of the anode.
Additionally, it was found that the current of photoelectrochemical
process was not only greater than the current of photocatalytic or electrochemical process
alone, respectively, but also greater than the sum of both current of photocatalytic and
electrochemical process alone. This may be an evidence of a synergic effect between
photochemical and electrochemical process in the reactor.
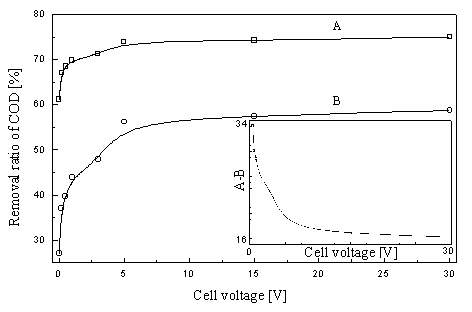
Fig.3
Dependence of voltage on COD removal
A--COD removal in the photoelectrochemical process; B--COD removal in the electrochemical
process;
3.2 Dependence of Voltage on COD
removal
The effect of applied voltage on the COD removal was conducted and the profile was shown
in Fig.3. It is obvious that both COD removal curves in the electrochemical and
photoelectrochemical processes increased dramatically with the increase of cell voltage.
However, the COD removal of the latter is always higher than that of the former.
Furthermore, the difference of their COD removal is also dependent on the applied voltage.
It is worth to notice that at the lower voltage, the differences of two curves become
greater. It means that there is a more significant synergic effect existing in the
photoelectrochemical process at lower voltage than at the higher voltage. The synergic
effect at the lower voltage may origin from the reduction of photo-generated electrons and
hole, not from other reactions because no any electrochemical reaction occurs at the lower
voltage.
The role of potential bias in the photocatalytic degradation of organic
pollutants is not only limited to reducing the electron-hole recombination, but also can
still have a direct or/and indirect electrochemical oxidation to the organic pollutant.
Thus, we could see from the curve B of Fig.3 that the COD removal of electrochemical
effect increased dramatically than that of photoelectrochemistry at the high cell voltage.
Therefore, it is convenient to control simultaneously the photocatalytic and
electrochemical reaction in the reactor under suitable voltage. This conclusion did not
conform to the Vinodgopal's view, he believed that it has not
been possible to influence a photoreaction in a TiO2 slurry system with an
externally applied potential bias [16].
3.3 Origin of Synergic Effect
It is convinced that oxygen could be changed into the stronger oxidizing agent, H2O2,
on the electrode by a two-electron reduction of oxygen on carbon electrode [17-18].
The electrogenerated H2O2 can be further changed into the OH· radical, in situ, by photolysis reaction in the presence
of UV light in the three dimension electrode-photocatalytic reactor [19]. Thus,
one of the degradation mechanisms of organic pollutants in the reactor is convinced to be
the OH·oxidation besides anodic oxidation. It is also
believed that the reaction of the photoeletro-generated OH·radical,
in situ, is an important origin of the above mentioned synergic effect of the COD removal
besides the recombining reduction of photogenerated- electron and -hole by anodic bias.
Moreover, OH·can be generated at the anode surface at the same time of oxygen
evolution [20-22]. These agents generated, in situ, can also cause the
oxidation of RBRX and its intermediates.
From the above analysis, the degradation of dye can be carried out by
many oxidation routines, such as anodic oxidation, the oxidation of electrogenerated H2O2
and OH·, the oxidation of photogenerated hole and OH·, and the photoelectrochemical synergic effect etc. This is
may be a good interpretation of effective destroying of RBRX using the three dimension
electrode-slurry photocatalytic reactor.
4. CONCLUSIONS
A new reactor, three dimension electrode-slurry photocatalytic reactor, was designed. It
has many advantages, such as not only has high efficiency of packed bed, but also has the
advantage of good mass transfer of fluid bed. Thus, the reactor has an apparent
characterization of current enhancement and high COD removal efficiency for pollutants.
REFERENCES
[1] Pelegrini R, Perqlta Z P, Andrade A R. Applied Catalysis B., 1999, 22: 83.
[2] Byrne J A, Eggins B R. J. Electroanal. Chem., 1998, 457 (1-2): 61.
[3] Vinodgopal K, Kamat P V. Chemtech., 1996, 26 (4): 18.
[4] Vinodgopal K, Hotchandani S, Kamat V. J. Phys. Chem., 1993, 97: 9040.
[5] Brillas E, Sauleda R, Casado J. J. Electrochem. Soc., 1997, 144: 2374.
[6] Bockris J O, Kim J J. Appl. Electrochem. 1997, 27: 890.
[7] Rajeshwar K, Ibanez J G, Swain G M. J. Appl. Electrochem., 1994, 24: 1077.
[8] Xiong Y, Karlsson H T. J. Environ. Sci. Health, Part A, 2001, 36 (3): Accepted.
[9] Kraeutler B, Bard A J. J. Am. Chem. Soc., 1978, 100 (7): 2239.
[10] Peterson M W, Turner JA, Nozik A J. J. Phys. Chem., 1991, 95 (1): 221-225.
[11] Bard A J. J. Photochem., 1979, 10 (1): 59-75.
[12] Vinodgopal K. J. Phys. Chem., 1994, 98 (27): 6797.
[13] Wahl A, Ulmann M, Carroy A. J. Chem. Soc., Chem. Commun., 1994, 19: 2277.
[14] Walker S A, Christensen P A, Shaw K E. J. Electroanal. Chem., 1995, 393 (1-2): 137.
[15] Haque I U, Rusling J F. Chemosphere, 1993, 26 (7): 1301.
[16] Vinodgopal K, Hotchandani S, Kamat V. J. Phys. Chem., 1993, 97: 9040.
[17] Tatapudi P, Fenton J M. J. Electrochem. Soc., 1993, 140: L55.
[18] Alverez G A, Pletcher D. Electrochim. Acta, 1999, 44 (14): 2483.
[19] Chen H, An T C, Fang Y J et al. Indian J Chem., 1999, 38B (7): 805-809.
[20] Brillas E, Sauleda R, Casado J. J. Electrochem. Soc., 1998, 145: 759.
[21] Canizares P, Dominguez J A, Rodrigo M A. Ind. Eng. Chem. Res., 1999, 38: 3779.
[22] Comninllis C, Battisti A D. J. Chim. Phys. Phys.Chim. Biol., 1996, 93: 673.