http://www.chemistrymag.org/cji/2001/039044le.htm |
Sep. 1,
2001 Vol.3 No.9 P.44 Copyright |
Combinatorial catalysis with physical, chemical and biological methodologies
Hu Wenxiang, Wang Jianying
(The Institute of Military Medicine, Headquarters of General Equipment, P.O.Box 9702,
Beijing 100101)
Received on May 16, 2001; Supported by National Sciences Foundation of China.
Abstract This paper reports the
application of physical and biological catalysis in organic or medicinal chemistry,
especially illustrates the advantages of microwave, ultrasonic wave, lipase catalysis and
combinatorial methodologies in organic synthesis or medicinal chemistry.
Keywords Physical catalysis, Biological catalysis, Combinatorial methodologies,
Synthesis
Organic synthetic chemistry has made rapid
progress during recent years. The new reactions, novel reagents, novel catalytic
methodologies and computer aided design of synthetic route have found wide application.
This made the complex reactions to be performed, easier and more economic. Application of
physical catalysis (mechanic methods, ultrasonic wave, microwave irradiation, x-ray, light
wave including electromagnetic wave of all wave band even laser, synchro-irradiation,
electronic field and magnetic field) and biological catalysis (esterase, lipase, protease,
abzymes, nucleicacidase, tissue, cell, yeast, microbe, "saccharinase" and "polymer enzyme" as we found in 1986) or mimic enzyme catalysis techniques can
speed up the rate of synthesis of organic compounds or pharmaceuticals, improve the
selectivity and increase yield of the reaction. So the whole appearance of reaction takes
on a good look[1-5].
Microwave, IR, UV, laser and ultrasonic wave catalysis techniques may
be called "high-energy"
techniques in organic synthesis. We have invented a new type of microwave reaction system
(CN P ZL 97201861). This instrument can be applied in organic, inorganic, medicinal,
analytical chemistry, molecular biology, material science and biological medicine. We have
used the set to catalyze esterification, ester exchange, alkylation, condensation and
Knovenagel reaction, and achieved good results. A new type of ultrasonic wave flux
reaction vessel (CN P ZL 97216451) has been used by us in organic reactions such as
addition, substitution, hydrolysis, esterification, transesterification, alkylation,
condensation, dehydrogenation, oxidation etc. In the meantime, this instrument has been
applied to the physical process of hair extraction, well-distributed disperse of detergent
and the well-distributed broken cell. The experiments mentioned above have given
satisfactory results.
Rapid reaction of pyrrole and benzaldehyde in xylene with microwave
irradiation afforded the tetraphenylporphyrin in good yield (about 30%) which was twofold
higher than that of dry reactions. Another advantage is the easiness of product
purification when compared with the normal methods.
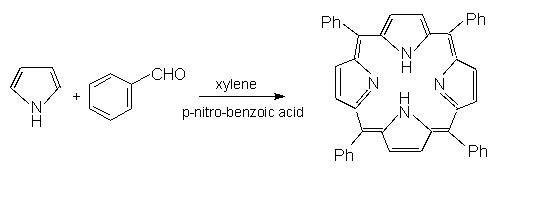
Scheme 1 The synthetic reaction of tetraphenylporphyrin by microwave irradiation
To the mixture of benzilic acid and methanol, was added drops of concentrated sulphuric acid and the reaction mixture was irradiated for 5 minutes under microwave (800 w) to afford 72.5% yield of methyl benzilate. The reaction product was identified by elemental analysis and spectroscopy. The reaction time is shortened by 100 times compared to the method of heating at reflux temperature, and the yield is increased by 30%. Successful closed-ring synthesis of pyridochromanone with the reaction of PPA and phenoxynicotinic acid by microwave irradiation for 3 minutes was reported here.
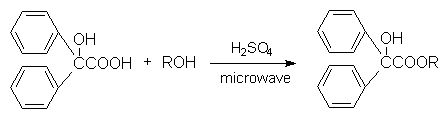

Scheme 2 The synthetic reactions of methyl benzilate and pyridochromanone by microwave irradiation
9-Anthraldehyde and 50% hydrazine hydrate were heated to reflux for 1 hour to produce 9-anthraldehyde hydrazone with the yield of 89.0% under ultrasonic wave catalysis. The transformation, however, could not take place on conventional heating condition even if the reactants were stirred for 48 hours. The synthesis of b-(1-Pyrene) hydrazone and b-(1-Pyrene) diazomethane was also accomplished with ultrasonic catalysis.
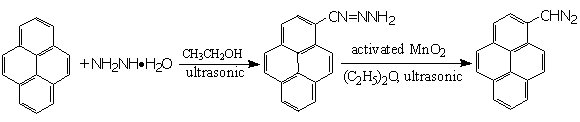
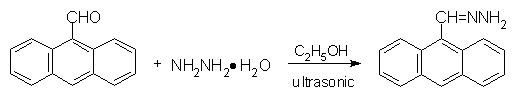
Scheme 3 Synthesis of 9-anthraldehyde hydrazone and b-(1-pyrene) diazomethane with ultrasonic
Enzyme is a type of biological catalyst[7-9]. Enzyme-catalyzed regioselective reactions, such as the transesterification catalyzed by lipase between benzilic acid and monose (D- glucose and D-fructose) or biose (sucrose, lactose and maltose) was performed respectively, and the results showed that the reaction yield of monose were better than that of biose. Owing to the different steric configuration of glucose and fructose, the esterifying positions is different, glucose afforded 6-position esterifying product while fructose afforded 1-position product. We tried to perform the above reactions with yeast instead of lipase, and the same results were achieved. This showed that yeast has some catalytic effect. Especially, when the ultrasonic wave catalysis combined with enzyme-catalyzed was utilized, the regioselective of transesterification of monose and biose can be enhanced and the yield increased from 5% by enzyme-catalyzed to 30% by combinatorial method, while the reaction time decreased from 6 days to 2 hours. The results indicated that combinatorial method have more advantages than single catalytic method. The combinatorial methodologies were also established among the physical, chemical and biological catalysis[10,11]. This catalytic mechanism may be explained by transition state theory including enthalpy and entropy effects.
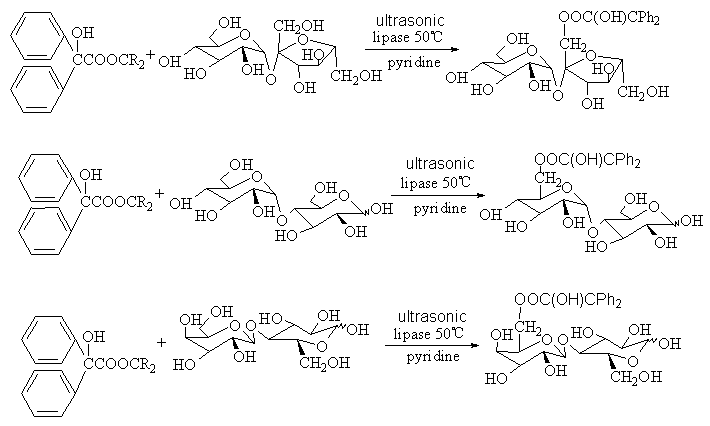
Scheme 4 The transesterification of sugars and benzilic with enzyme-catalyzed
The evidence above indicate that applying physical, biological or chemical catalysis and their combinatorial methodologies to synthesis, not only can greatly speed up the reaction rate and simplify the purification procedure, but also increase the yield and improve the selectivity of reaction. The following ten kinds of combinatorial methodologies, ultrasonic-enzyme-catalyzed, ultrasonic- enzyme- chemical- catalyzed, microwave-chemical-catalyzed, electronic field- ultrasonic- chemical catalyzed, electronic field- quaternaries- enzyme- catalyzed, infrared-chemical-catalyzed, ultrasonic-chemical-catalyzed, electronic field- chemical- catalyzed, magnetic field- chemical- catalyzed and enzyme- chemical- catalyzed, combined with new experimental tools, would replace and improve the concept of conventional organic synthesis, including combinatorial chemistry. And the related chemical industry, pharmaceuticals, biomedicines and national defense would gain a great advantage in the new century.
REFERENCES
[1] Hu W X, Yun LH. Chin. J. Med. Chem. (Zhongguo Yaowu Huaxue Zazhi), 1993, 3: 76-78.
[2] Hu W X, Yuan C Y, Li S. Chin. Chem. Lett. (Zhongguo Huaxue Kuaibao), 1992, 3: 167-170.
[3] Hu W X, Yuan C Y. Acta Chemica Sinica (Kexue Tongbao), 1996, 54: 77-83.
[4] Michel T, Klibanov A M. J. Am. Chem. Soc., 1987, 109: 3977-3983.
[5] Lu M W, Hu W X, Yun L H. Chin. J. Org. Chem. (Youji Huaxue), 1995, 15: 561-566; 1997,
17: 289-294.
[6] Petit A, loupy A, et al. Synth. Commun, 1992, 22 (8): 1137-1141.
[7] Hu W X, Yun L H. Chin. Bull. Sci.Technol (Keji Tongbao), 1996, 12 (5): 320-321.
[8] Gao S H, Hu W X. Chin. J. Appl. Environ. Biol. (Yingyong Huanjing Shangwu Xuebao),
1996, 2 (4): 415-423.
[9] Hu W X. Chemisry (Huaxue Tongbao), 1995, (8): 35; 1999, (10): 34.
[10] Li W Z, Yun L H, Hu W X. Symposium on Frontiers of Chemistry in Conjunction with the
Second Conferences for Worldwide Chinese Young Chemists, Hong Kong, 1997
[11] Hu W X. Scientific American in Chinese (Kexue), 1999, (12): 9-11; 2000, (1): 54-57.