http://www.chemistrymag.org/cji/2001/03a051nc.htm |
Oct. 1,
2001 Vol.3 No.10 P.51 Copyright |
Wang Yangang,Lu Bingxi, Yu Xiaoyuang, Ye Wenfa,Wang Sheng
(Department of Chemistry, Central China Normal University, Wuhan, 430079, China)
Abstract Sixteen new Schiff bases of
tetrazole have been synthesized for the first time.Their Structures were originally
characterized by UV,IR,1HNMR and elementary analysis.It is found from the
results of biological tests that some compounds have remarkeble activity on plant hormone,
Ia, Id, Ik, Il, In, Ip
have a good activity on auxins, Ic, Id, Ie, Ig,
Ih, Il have a excellent activity on growth of root.In particalar
Schiff bases of 2-chlorobenzaldehyde, 4-methylbenzal -dehyde, thiophenaldehyde and
indolaldehyde have more excellent auxins activity.
Keywords Tetrazole,Schiff base,Synthesis,Plant hormone.
5-氨基四唑Schiff碱的合成与植物激素活性研究
汪焱钢 芦冰熙 禹筱元 叶文法 王 胜
(华中师范大学化学系,武汉 430079)
2001年4月6日收稿; 国家自然科学基金资助项目(批准号:20072009)
摘要 合成了16个新的含四唑环的Schiff碱,采用UV、IR、1HNMR及元素分析确定了它们的结构,生物活性测试表明,其中一些化合物表现出明显的植物激素活性,Ia, Id, Ik, Il, In, Ip等化合物具有好的生长素活性,Ic, Id, Ie, Ig, Ih, Il具有优异的生根性能。尤其是2-氯苯甲醛、4-甲基苯甲醛、噻吩醛和吲哚醛的Schiff碱具有更好的生长素活性。关键词 四唑,Schiff碱,合成,植物激素
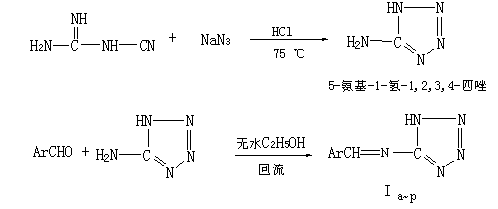
一些四氮唑的衍生物有优良的抗微生物活性及消炎作用[1-3],张自义等人的研究工作发现含四氮唑环的氨基硫脲等具有良好的植物生长调节活性[4],前文报道了5-氨基三氮唑-3-羧酸及2-氨基-5-巯基-1,3,4-噻二唑的Schiff碱的合成及植物激素活性[5,6],而含四唑环的Schiff碱还未见报道。我们采用5-氨基-1-氢1,2,3,4四唑与芳醛或杂环醛反应,合成了16个尚未见报道的含四唑环的Schiff碱,并进行了室内生物活性测试,发现其中不少化合物具有优良的植物激素活性,尤其是生长素活性与生根性能更好。该系列化合物的合成路线如下:
Ar- 4-CH3C6H4- 4CH3OC6H4- 3-CH3O-4-C2H5OC6H3- 2-ClC6H4- 4-ClC6H4- Compd If Ig Ih Ii Ij
Ar- 2-Cl-4-ClC6H3- 2-Cl-3-ClC6H3- 2-NO2C6H4- 3-NO2C6H4- 4-NO2C6H4- Compd Ik Il Im In Io Ip
Ar- 4-OHC6H4- 2-CH3O4OHC6H3
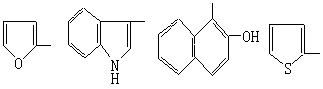
1 实验部分
PE-983型IR光谱仪(KBr压片),岛津UV-265型紫外分光光度计,XL-200MHz核磁共振仪(TMS为内标,溶剂为DMSO-d6),PE-2400型元素自动分析仪,显微熔点测定仪,所用药品均为化学纯试剂。
1.2 5-氨基-1-氢-1,2,3,4-四唑的合成
在三口烧瓶中加入210g水,0.05mol二氰二胺,0.1mol叠氮化钠,升温至75℃,在约3h滴加完9ml浓盐酸,使pH为3.1再继续反应2h,冷却,析出白色片状结晶,抽滤得粗产品,在水溶液中重结晶,得5-氨基四唑纯品。
1.3 5-氨基四唑Schiff碱的合成[7,8]
在圆底烧瓶中加入0.01mol 5-氨基四唑与0.01mol芳醛,无水乙醇作溶剂,加热回流3~4h,冷却,析出结晶,用无水乙醇洗涤粗产品,再用无水乙醇重结晶,得纯品Ia~Ip。
2 结果与讨论
2.1 分析结果
目标化合物Ia~p产率、物理性质和元素分析见表1,其波谱数据见表2。 Table 1 Physical properties,Yield and data of elementary analysis for compound
| Compd | Formula |
Yield |
m.p/ (℃) |
Elemenlary analysis(%,calcd) |
||
C |
H |
N |
||||
Ia |
C9H9N5 |
50.0 |
188~189 |
57.95(57.76) |
4.82(4.80) |
37.63(37.44) |
Ib |
C9H9N5O |
53.2 |
157~158 |
53.25(53.20) |
4.40(4.43) |
34.42(34.48) |
Ic |
C11H13N5O2 |
63.0 |
195~196 |
53.59(53.44) |
5.32(5.26) |
28.39(28.45) |
Id |
C8H6ClN5 |
72.5 |
186~188 |
46.09(46.15) |
2.95(2.88) |
33.76(33.65) |
Ie |
C8H6ClN5 |
81.5 |
188~189 |
46.25(46.15) |
2.84(2.88) |
33.52(33.65) |
If |
C8H5Cl2N5 |
80.0 |
172~173 |
39.80(39.66) |
2.12(2.07) |
28.90(28.93) |
Ig |
C8H5Cl2N5 |
78.0 |
162~163 |
39.58(39.66) |
2.15(2.07) |
28.88(28.93) |
Ih |
C8H6N6O2 |
74.5 |
126~128 |
44.07(44.04) |
2.69(2.75) |
38.61(38.53) |
II |
C8H6N6O2 |
78.0 |
161~162 |
44.12(44.04) |
2.81(2.75) |
38.49(38.53) |
Ij |
C8H6N6O2 |
80.0 |
186~188 |
44.10(44.04) |
2.78(2.75) |
38.60(38.53) |
Ik |
C8H7N5O |
40.0 |
192~194 |
50.72(50.79) |
3.82(3.70) |
37.01(37.04) |
Il |
C9H9N5O2 |
55.0 |
189~190 |
49.24(49.31) |
4.13(4.11) |
31.91(31.96) |
Im |
C6H5N5O |
57.5 |
166~168 |
44.24(44.17) |
3.01(3.07) |
42.78(42.94) |
In |
C10H8N6 |
54.1 |
186~187 |
56.55(56.60) |
3.87(3.78) |
39.58(39.62) |
Io |
C12H9N5O |
45.5 |
200~202 |
60.31(60.25) |
3.83(3.77) |
29.42(29.29) |
Ip |
C6H5N5S |
41.1 |
210~211 |
40.10(40.22) |
2.83(2.79) |
39.08(39.11) |
从表2可以看出,目标化合物的紫外吸收明显红移,说明Schiff碱体系共轭效应增强,芳醛环上连有羟基、烷氧基等供电子基,其紫外吸收多在330nm左右,尤其是2-羟基萘甲醛的Schiff碱紫外吸收高达380nm。1HNMR谱表明,四唑环上的N-H,其化学位移为10左右,这么高的化学位移说明四唑环具有强的吸引电子效应和较强的去屏蔽效应。双键中质子的化学位移值在9.1~9.6之间,也进一步说明了该键两边的芳环和四唑环具有强的去屏蔽效应。
Table 2 Data of UV、IR and 1HNMR compoundsIa~p| Compd | UV lmax/nm |
IR >C=N- |
1HNMR(DMSO-d6),δ |
| Ia | 295 | 1605 | 10.0(S,1H,tetrazole),9.2(S,1H,-CH=N-),7.5~8.1 (m, 4H,PhH),1.2(S,3H,-CH3) |
| Ib | 311 | 1625 | 9.8(S,1H,tetrazole),9.1(S,1H,-CH=N-),7.1~8.1(m,4H,PhH), 3.8(S,3H,CH3O-) |
| Ic | 335 | 1635 | 9.9(S,1H,tetrazole),9.2(S,1H,-CH=N-),7.1~7.7(m,3H, PhH) ,3.8~4.2(5H,C2H5O-) |
| Id | 280 | 1615 | 10.0(S,1H,tetrazole),9.3(S,1H,-CH=N-),7.7~8.2(m,4H,PhH) |
| Ie | 282 | 1610 | 10.2(S,1H,tetrazole),9.3(S,1H,-CH=N-),7.7~8.2(m,4H,PhH) |
| If | 283 | 1625 | 10.2(S,1H,tetrazole),9.5(S,1H,-CH=N-),7.5~8.3(m,4H,PhH) |
| Ig | 283 | 1620 | 10.2(S,1H,tetrazole),9.5(S,1H,-CH=N-),7.5~8.2(m,4H,PhH) |
| Ih | 280 | 1615 | 10.2(S,1H,tetrazole),9.6(S,1H,-CH=N-),7.6~8.3(m,4H,PhH) |
| Ii | 279 | 1610 | 10.2(S,1H,tetrazole),9.4(S,1H,-CH=N-),7.7~8.4(m,4H,PhH) |
| Ij | 280 | 1605 | 10.0(S,1H,tetrazole),9.5(S,1H,-CH=N-),7.7~8.0(m,4H,PhH) |
| Ik | 333 | 1640 | 10.1(S,1H,tetrazole),9.4(S,1H,-CH=N-),8.0~8.4(m,4H,PhH),9.6(S,1H,HO-) |
| Il | 335 | 1630 | 9.9(S,1H,tetrazole),9.2(S,1H,-CH=N-), 7.1~7.6(m,3H,PhH),3.84(S,3H,CH3O-) |
| Im | 309 | 1605 | 9.8(S,1H,tetrazole),9.1(S,1H,-CH=N-),7.5~8.2(m,3H,Furane) |
| In | 320 | 1620 | 12.0(S,1H,tetrazole),9.9(S,1H,-CH=N-),7.2~8.3(m,6H,Indole) |
| Io | 380 | 1605 | 10.9(S,1H,tetrazole),10.0(S,1H,-CH=N-),7.3~8.8(m,6H,Naphthalene),9.4(S,1H,-CH=N-) |
| Ip | 317 | 1610 | 9.9(S,1H,tetrazole),9.4(S,1H,-CH=N-),6.5~8.0(m,3H,Thiophene) |
| Compd | Auxins activity | Cytokinin activity | Growth of root | |||
| Effect(%) | Grade | Effect(%) | Grade | Effect(%) | Grade | |
| Ia | 35.3 | A | 1.1 | D | 87.5 | C |
| Ib | 29.4 | B | 7.1 | D | 2.5 | D |
| Ic | 31.6 | B | 14.3 | C | 162.5 | A |
| Id | 35.1 | A | 21.4 | C | 175.0 | A |
| Ie | 31.3 | B | 14.3 | C | 50.0 | A |
| If | 31.4 | B | 28.6 | B | 110 | B |
| Ig | 23.5 | C | 0.8 | D | 150 | A |
| Ih | 19.6 | C | 17.9 | C | 150 | A |
| Ii | 29.4 | B | 16.8 | C | 62.5 | C |
| Ik | 32.3 | B | -3.6 | D | 0 | D |
| Il | 31.4 | B | -7.1 | D | 137 | A |
| In | 35.3 | A | 0.6 | D | 150 | A |
| Ip | 37.2 | A | 14.3 | C | 97.5 | B |
| IAA* | 35.3 | 125 | ||||
| KT** | 39.3 | |||||
| CK | 0 | 0 | 0 | |||
2.2 生物活性
对目标化合物进行了室内生物活性测试,采用小麦芽鞘法测定生长素活性,采用黄瓜子叶法测定细胞分裂素活性,用黄瓜子叶生根试验测定其生根性能,药液浓度均为10mg/L,测试方法见文献[5]。生物活性测试结果见表3。
从表3可看出,含四唑环的Schiff碱大多具有明显的植物激素活性,尤其是生长素活性较优,而部分化合物则具有优良的生根性能,其中以吲哚醛、噻吩醛与2-氯苯甲醛的Schiff碱活性最好,其生长素活性和生根性能已达到或略超过常用的吲哚乙酸。
[1] Peet N P, Baygh L E, Sunder S et al. J. Med. Chem., 1986, 29: 2403.
[2] Peet N P, Sunder S, Barbuch R J et al. J. Hetrocyclic Chem., 1987, 24: 1531.
[3] Jose L C, Richard G B. J.Med.Chem., 1996, 39: 842.
[4] Zhang Ziyi, Yang Hu. Chinese J.Org. Chem.(Youji Huaxue), 1986, 3: 184.
[5] Wang Yangang, Cao Lei, Yang Jun et al. Chem.J.Chinese Universities (Gaodeng Xuexiao Huaxue Xuebao), 1998, 19 (8): 1247.
[6] Wang Yangang, Cao Lei, Yang Jun et al. Chem.J.Chinese Universities (Gaodeng Xuexiao Huaxue Xuebao), 1999, 20 (12): 1903.
[7] Miller A E, Eecnty D J, Yan Ma. Synthetic Communications, 1990, 20 (2): 217.
[8] Huff B E, Staszak M A. Tetrahedron Letters, 1993, 34: 8011.