http://www.chemistrymag.org/cji/2001/03b054pe.htm |
Nov. 1,
2001 Vol.3 No.11 P.54 Copyright |
Photochemical surface modification of polyimide containing benzophenone unit by UV light source
Zhu Chengxiang, Lu Qinghua, Yin Jie
(Research Institute of Polymer Material, School of Chemistry and Chemical Engineering,
Shanghai Jiao Tong University, 200240)
Supported by Ministry of Education of China, Shanghai Municipal Science and Technology Commission ("Qi Ming Xing" Project) and National Natural Science Foundation of China (Grant No. 20004006).
Abstracts Since polyimide has been found
a wealth of applications in many Hi-Tech sections, its surface modification has been
attracting much research interest trying to alter this relatively inactive surface. Many
chemical and physical approaches are invented to modify the polyimide surface, such as
chemical vapor deposition and reactive electron beam etching. In this research, a
photochemical means has been applied to achieve the surface modification of polyimide
(BTDA/ODA) containing benzophenone unit in its main chain. Acrylic acid, as the modifying
monomer, is fixed onto the polyimide surface by using a pre-immersing process and the
carboxylic group in the monomer allows the potential of doing more chemistry. To
investigate the mechanism of this process, two models are put forward and various methods
such as FTIR and ESR are utilized to characterize the modified film, the substrate surface
radical initiation mechanism is then excluded. A conclusion was drawn that
penetrating-self-polymerization was the mechanism leading to this modification.
Keywords polyimide, surface modification, ultraviolet light, acrylate
1 INTRODUCTION
The surface photografting of polymers emphasizes the alteration of surface properties such
as hydrophilicity, surface energy, adhesivity, etc. so as to endow materials with new
values while maintaining their bulk properties. Polyimide has been found more and more
applications in many Hi-Tech fields owing to its outstanding comprehensive properties. In
the area of surface modification of polyimide, increasing concern is focused on the ways
to improve the adhesion between polyimide and metals or other polymers. A great number of
articles have been published demonstrating various approaches to modify the relatively
inactive polyimide surface, e.g. typical wet-chemistry ways such as hydrolysis[1]
and oxidation[2], chemical vapor deposition[3], electron beam
modification[4-5], plasma bombardment[6], excimer laser radiation[7-8]
etc. Some of these methods have reached commercial maturity. Surface photografting is a
convenient method to achieve surface modification and patterning. As a non-touch approach
based on wet photochemistry, it requires low-cost apparatus and simple process control,
indicating great commercial potential. However, only mass-produced plastics such as PP,
HDPE, LDPE were used as the substrates in the existing surface photografting reports. No
study was undertaken on photografting of polyimide surface. Furthermore, lots of
researchers add benzophenone as photosensitizer to initiate the surface modification
process. What it will be if a substrate containing benzophenone unit in the molecule is
used? In this study, we will describe a photografting process on polyimide surface aided
by UV radiation, benzophenone unit is contained in the polymer main chain.
2 EXPERIMENTAL
2.1 Material preparation
The structure of the polyimides used in this study is shown below:
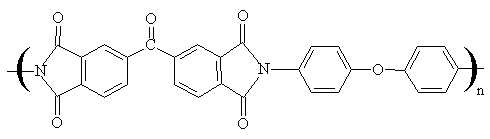 Polyimide I
Polyimide I ¡¡
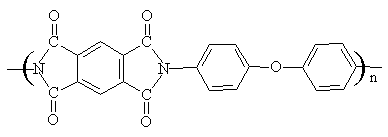 Polyimide II
Polyimide II
Fig 1 Structures of two polyimides
Condensation polymerization took place
between 4,4'-oxydianiline(ODA) and corresponding dianhydrides (3,3'
4,4'-benzophenonetetracarboxylic dianhydride, BTDA, for polyimide I) and (pyromellitic
dianhydride, PMDA, for polyimide II) in N-methyl 2-pyrrolidine(NMP) solution, the
resulting solution was then spin-coated onto a glass slide to form a thin and even film
which was gradually heated to finish imidization. Acrylic acid (Shanghai No.1 Reagent
Factory) was distilled under reduced pressure to remove the inhibitor. Stearyl acrylate
(Beijing Oriental Acrylate Co. Ltd) was washed with ethanol to remove the inhibitor.
2.2 Sample preparation
Some films were immersed in acrylic acid solution (0.5M, acetone solution) or stearyl
acrylate solution (0.5M, cyclohexane solution) for 48 hours, a quartz plate was then
applied on the immersed film. At ambient temperature, this assembly was irradiated for a
given period of time under UV light (the sample was put on a support with constant
temperature which is 30cm away from the 1000W lamp). The quartz plate was removed after
12hrs¡¯ extraction with acetone. The film
subsequently subjected to solvent rinsing and another 12hrs¡¯ extraction before it was dried under vacuum.
Other films were irradiated under UV light immediately after one drop
of acrylic acid was applied onto the surface and covered with quartz plate. The following
procedure was the same as those films immersed.
2.3 Characterization
FTIR analysis was undertaken on a BRUCKER EQUINOX 55 IR
spectrophotometer and a PERKIN ELMER PARAGON1000 spectrophotometer. ESR spectrum was
measured on a JES-FEIXG immediately after UV radiation on a film without immersion in
advance. The magnetic intensity scanned from 2700 Gauss to 3700 Gauss, the microwave
frequency was 4295MHz.
3. RESULTS AND DISCUSSION
3.1 Effect of the pre-immersing process
As shown in Fig 2, difference is evident in the FTIR spectra before and after UV radiation on the immersed polyimide I films. Acrylic acid is used in this experiment as the modifying monomer. A strong and broad peak appears between 3600cm-1 and 2300cm-1 after the radiation, consistent with the carboxylic group in the poly(acrylic acid). The small peak at about 2900cm-1 should be owed to the methylene stretching. The sharp absorption at 1600-1700cm-1 refers to the stretching vibration of the carbonyl group. This FTIR spectrum is highly consistent with the document[9] . Further evidences for surface modification include increased surface adhesion, pale shade and reduced surface smoothness etc. It is therefore demonstrated that the poly(acrylic acid) macromolecules have been grafted onto the surface of polyimide film.
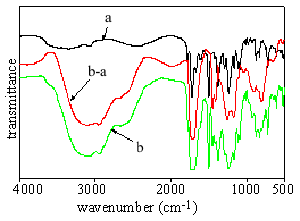 |
Fig 2 FTIR spectra
of immersed polyimide films before and after UV radiation and their difference a: before radiation; b: after radiation; b-a: the difference between a and b |
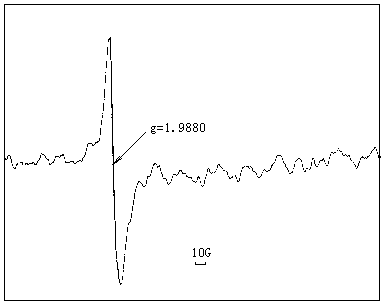 |
Fig 3 ESR spectrum when film irradiated by UV light |
According to the results of existing literature[10-12], the p electron in benzophenone unit of polyimide I should undergo p-p* transition upon UV radiation and is converted to the singlet state(S1), the ¡°short-lived¡± S1 further transits to ¡°long-lived¡± tripet1(T1) via intersystem crosslinking(ISC). After that, a radical is formed on the polymer chain through hydrogen abstraction. The ESR measurement demonstrates the existence of radicals in polyimide I upon UV radiation, as shown in Fig 3. However, the fact that this signal can still be detected after five days indicates the inertness of the radicals, therefore, it is quite doubtful that so inactive species is able to initiate chain propagation.
The FTIR spectra in Fig 4 do not indicate any significant change when comparing the film radiated immediately after a drop of acrylic acid solution is applied onto polyimide I surface. This phenomenon is unusual since it contradicts with the existing reports if the surface radicals generated through the above-mentioned process are active enough.
In principle, surface modification could proceed either a surface-initiating or a penetrating-self-polymerization mechanism in the particular system in the present investigation: the former means the carbonyl group in BTDA is excited upon UV radiation, leading to the formation of a radical which has the ability to initiate chain propagation, this process proceeds very fast and has no requirement on the size of monomer molecule; the latter means the monomer, with its small molecular size, penetrates into the film surface during the course of immersion, and forms macromolecules upon the transmitted UV light, this means only small monomer molecules can achieve this process and it takes relatively longer time. These two ways are schematically shown in Fig 5.
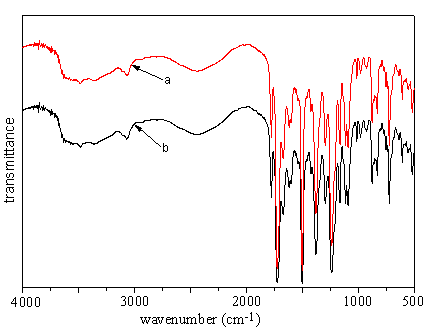 |
|
| Fig 4 FTIR spectra
before and after UV radiation on the film without immersion process (a) before radiation; (b) after radiation |
Fig 5 two possible surface modification processes |
The molecule of stearyl acrylate is much larger than acrylic acid. To investigate whether or not the molecular size has an effect on surface modification, another experiment is taken using stearyl acrylate solution on polyimide I surface. No absorption for methylene stretching vibration is observed in both spectra (Fig 6) of the immersed films. This indicates that no stearyl acrylate is grafted onto the film. However, in both cases of acrylic acid and stearyl acrylate, viscous substance is found between the quartz plate and the polymer film, indicating that both monomers are capable of self-polymerizing to form macromolecules upon UV radiation. Here it could be inferred that the difference lies in the molecular size of the two monomers: during the course of immersion, acrylic acid is able to penetrate into the polyimide surface while stearyl acrylate is not.
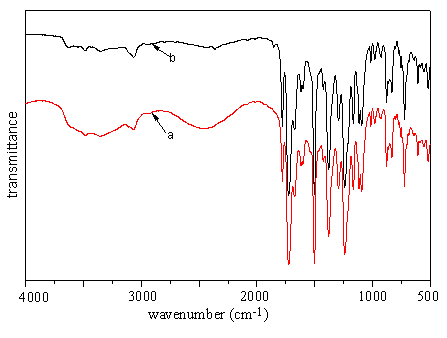 |
Fig 6 FTIR spectra of polyimide
film immersed in stearyl acrylate solution (a) before radiation (b) after radiation |
A designed
comparative experiment provides some more evidence. Compared with polyimide I, polyimide
II contains no benzophenone unit in its main chain and shows no radical signal in ESR
spectrum. Through the same process as described in the experimental section, substantial
changes take place in the ATR-IR spectra (Fig 7). The polyimide surface before radiation
is quite smooth and contacts the crystal in the ATR assembly closely. Due to the less
smooth surface after the reaction, such a close contact is hard to achieve, as causes
energy loss in reflection, the baseline value of curve b is therefore much larger than
that of curve a, yet the methylene, carbonyl stretching vibration can still be found in
the spectrum. This indicates that the benzophenone unit in the polymer main chain is
unnecessary to make surface modification happen. With these facts, it could be concluded
that this process was achieved via a penetrating-self-polymerization mechanism in the
particular system in this study.
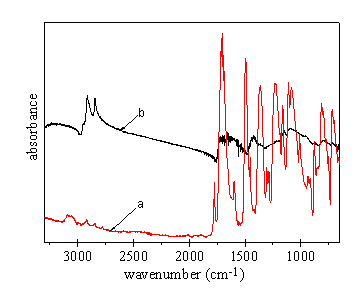 |
Fig 7
ATR-IR spectra of polyimide II films before and after UV radiation |
4. CONCLUSION
We have demonstrated that acrylic acid could be introduced onto the polyimide surface by
using the "pre-immersing" process using FTIR
spectra data. However, the benzophenone unit in polyimide I is not the origin to the
change in surface properties, instead, the results are achieved via a
penetrating-self-polymerization process. The result is a fairly interesting one since the
carboxylic acid group in the poly(acrylic acid) allows further chemistry to be associated
to the surface.
REFERENCE
[1] Thomas R, Buchwalter L P, Stephen L et al. Macromolecules, 1992, 25 (18): 4559-4568.
[2] Ghosh I, Konar J, Bhowmick A K. Journal of Adhesion Science and Technology, 1997, 11
(6): 877-893.
[3] Jeon N L, Nuzzo R G. Langmuir, 1995, 11 (1): 341-355.
[4] Choi S C, Koh S K. Proceedings of the International Conference on Ion Implantation
Technology, 1998 1999, Jun, 1078-1081.
[5] Tsang Y L, Miller C, Lii T. Journal of the Electrochemical Society, 1996, 143 (4):
1464-1469.
[6] Chin J W, Wightman J P. SAMPE Quarterly, 1992, 23 (2): 2-10.
[7] Ikegame T, Murahara M. Materials Research Society Symposium ¨C Proceedings, 1998 1999, Nov-Dec, 227-232.
[8] Hiraoka H, Lazare S. Applied Surface Science, 1990, 46 (1-4): 264-271.
[9] Ranby B, Yang W T, Tretinnikov O, Nuclear Instruments and Methods in Physics Research
B, 1999, 151 (1-4): 301-305.
[10] Ranby B. Polymer Engineering and Science, 1998, 38 (8): 1229-1243.
[11] Ranby B. Journal of Adhesion Science and Technology, 1995, 9 (5): 599-613.
[12] Yang W T, Ranby B. European Polymer Journal, 1999, 35 (8): 1557-1568.¡¡
¡¡