http://www.chemistrymag.org/cji/2001/03b055pe.htm |
Nov. 1,
2001 Vol.3 No.11 P.55 Copyright |
Shan Jinhuan, Wei Haiying, Wang Li, Shen
Shigang, Liu Baosheng, Sun Hanwen
(College of Chemistry & Environmental Science, Hebei University, Baoding 071002 China)
Abstract The kinetics of oxidation of ethyleneglycol monobutylether(EGB) by dihydroxydiperiodatonickelate
(IV) complex(DPN) in aqueous alkaline medium at a temperature range of 25-45oC was studied by spectrophotometry. The reaction was found to be first order with respect to both Ni(IV) and EGB. The rate increased with the increase in [OH-] and decreased with the increase in [IO4-]. Added salts did not affect the rate and no free radical was detected. In view of these, a plausible mechanism of reaction involving a rapid preequilibrium is proposed. In addition, the rate equation which is derived from the mechanism can explain all experimental observations. Activation parameters of the rate-determining step were calculated.Keywords dihydroxydiperiodatonickelate(IV), ethyleneglycol monobutylether, redox reaction, kinetics and mechanism
In recent years, study of the highest
oxidation state of transition metals intrigued many researchers' interests, which can
provide new and valuable information in some fields.
Transition metals, in a higher oxidation state, can generally be
stabilized by chelation with suitable polydentate ligands. Metal chelates such as
diperiodatocuprate(III)[1], diperiodatoargentate(III)[2],
diperiodatonickelate
1. EXPERIMENTAL
1.1 Materials
All reagents used were of A.R. grade. All solutions were prepared with twice-distilled
water. Solutions of DPN and EGB were always freshly prepared before using with stock
solution and twice-distilled water. The stock solution of DPN in a strong alkaline medium
was prepared by the procedure given by Baker[8] and standardized by the method
by Murthy[9]. Its electronic spectrum was found to be consistent with that
reported by Murthy.
1.2 Kinetic measurement and reaction product analysis
The kinetic measurement was described elsewhere[10].The product of oxidation
was the corresponding aldehyde by its characteristic spot test[11].
2. RESULTS AND DISCUSSION
2.1 Evaluation of pseudo-first order rate constants
2.2 Rate dependence on [EGB]
At fixed [Ni(IV)], [OH-], [IO4-], ionic strength I and temperature, kobs increased with the increase of the [EGB]. Furthermore, the plots of kobs vs. [EGB] were linear through the origin at different temperatures (Fig 1), indicating the reaction order dependence on EGB was first order.
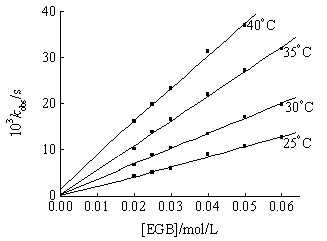
[Ni(IV)]=1.4854×10-4mol/L; [IO4-]=1.8378×10-3mol/L;[OH-]=1.3541×10-2mol/L; I=0.01651 mol/L
2.3 Rate dependence on [OH-]
2.4 Rate dependence on [IO4-] and ionic strength I
At fixed [Ni(IV)], [EGB], [OH-], I and temperature, kobs decreased with the increase of [IO4-]. The order of IO4- was negative fractional (nap=-0.31) and the plot of 1/kobs vs. [IO4-] was linear (Table 1), showing that there was a preequilibrium involving the process of disassociation H2IO63-from Ni(IV)complex. At fixed conditions except ionic strength I, kobs hardly altered with the increase in I (Table 1).
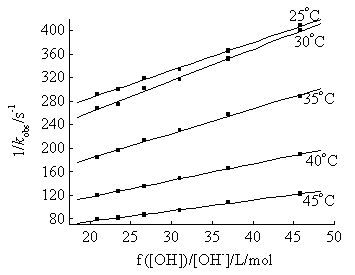
Fig. 2 The plots of 1/kobs vs. f([OH-])/[OH-]
at different temperatures
[Ni(IV)]=1.613×10-4mol/L; [IO4-]=1.79×10-3mol/L;
[EGB]=2.50×10-2mol/L; I=1.636×
102I/mol/L |
103 [IO4-]/mol/L |
103kobs/s-1 |
1.636 |
1.79 |
7.446 |
2.940 |
1.79 |
7.893 |
4.256 |
1.79 |
8.253 |
5.721 |
1.79 |
8.212 |
6.636 |
1.79 |
8.284 |
1.636 |
2.79 |
6.507 |
1.636 |
3.79 |
5.93 |
1.636 |
4.79 |
5.576 |
1.636 |
5.79 |
5.098 |
[Ni
(IV)]=1.613×10-4mol/L; [EGB]=2.50×10-2mol/L;[OH-]=1.313×10-2mol/L2.5 Free radical detection
The addition of acrylonitrile or acrylamine to the reaction mixture under the protection
of nitrogen neither changed the rate nor there was any polymerization, which showed the
absence of free radical in the reaction.
2.6 Discussion
In aqueous periodate solution, equilibria (1)-(3) were detected and
the corresponding equilibrium constants at 25
IO4- +OH- + H2O
IO4- + 2OH-
The distribution of all periodate species
in aqueous solution was calculated from equilibria (1)-(3) . The dimer(H2I2O104-)
and IO4- species of periodate can be neglected. The main species of
periodate are H3IO62- and H2IO63-,
consistent with the result calculated from Crouthamel's data[13] by Murthy.
Based on such distribution, the formula of Ni(IV) periodate complex may be
represented by either [Ni(OH)2(H3IO6)2]2-
or the less protonated [Ni(OH)2(H2IO6)2]4-.
We preferred to use the latter to represent DPN because it is close to that suggest by
Mukherjee[14] and will obtain support from kinetic studies.
In view of the above results and discussion, a plausible reaction
mechanism was proposed:
DPN MPN
[Ni(OH)2(H2IO6)]2- + HOCH2CH2OC4H9MPN
adduct Here, reaction (5) was
the rate-determining step.
As the rate of the disappearance of Ni(IV) was monitored, the rate of
the reaction can be derived as:
Subscripts T and e stand for total concentration and concentration at equilibrium respectively. Neglecting the concentration of ligand dissociated from Ni(IV) and the species of periodate other than H2IO63- and H3IO62-, equations (9) and (10) can be obtained from (2) and (3):
Here [IO4-]ex represents the original overall entering periodate and equals approximately to the sum of [H2IO63-] and [H3IO62-].
Substituting eq.(9) into (8), we can get the following equations:
If the formula of DPN was [Ni(OH)2(H3IO6)2]2-, getting eq. (10) into (8) obtained eq.(13):
Table 2 Rate constants, equilibrium constant and activation parameters of rate-determining step
Constants |
T/K |
Activation parameters at 298.2K |
||||
298.2 |
303.2 |
308.2 |
313.2 |
318.2 |
||
k/mol/L/s-1 |
0.2096 |
0.2605 |
0.4034 |
0.6516 |
1.017 |
Ea*=63.88kJ/mol |
K |
0.0725 |
0.0513 |
0.0423 |
0.0393 |
0.0388 |
|
* r=0.992, a=24.08, b=-7683.63 for the linear regression of lnk vs. 1/T.
The plots of 1/kobs vs. f([OH-])/[OH-] were linear at different temperatures. From their intercepts, the rate-determining step constants k were evaluated. The activation parameters data were calculated[15] (Table 2).
REFERENCES
[1] Niu W J, Zhu Y, Hu K C et al. Int. J. Chem. Kinetics,1996, 28 (12): 899.
[2] Shi T S. Science in China(series B) (Zhongguokexue B), 1990, 33: 471.
[3] Chandraiah U, Murthy C P, Sushama K. Indian J. of Chem. 1989, 28A: 162.
[4] Santanu B, Pradyot B. Bull. Chem Soc. Jpn., 1996, 69: 3475.
[5] Li Z T, Wang F L, Wang A Z. Int. J. Chem. Kinetics, 1992, 24: 933.
[6] Halligudi N N, Desai S M, Nandibewoor S T. Int. J. Chem. Kinetics, 1999, 31: 789.
[7] Li Z T, Chang Q, Li B W, et al. Chinese Research in Chinese Universities
(Gaodengxuexiao Huaxuexuebao), 2000, 21 (5): 747.
[8] Baker L C W, Mukherjee H G, Sarkar S B et al.. Indian J. Chem., 1982, 21A: 618.
[9] Murthy C P, Sethuram B, Rao T. Z. Phys. Chem.(Leipzig), 1986, 287: 1212.
[10] Shan J H, Qie L J, Guo X S. Acta Chimical Sinica (Huaxue Xuebao), 1997, 55: 458.
[11] Feigl F. Spot Tests in Organic Analysis, New York: Elsevier Publishing Co., 1956.
[12] Aveston J. J. Chem. Soc.(A), 1969: 273.
[13] Crouthamel C E, Meek H V, Martin D S et al. J. Am. Chem. Soc., 1949, 71: 3031; 1951,
73: 82.
[14] Mukherjee H G, Mandal B, De S. Indian J. Chem., 1984, 23A: 426.
[15] Shan J H, Liu T Y. Acta Chimica Sinica (Huaxue Xuebao), 1994, 52: 1140.