http://www.chemistrymag.org/cji/2003/051002ne.htm |
Jan. 1, 2003 Vol.5 No.1 P.2 Copyright |
(Key laboratory of analytical science and technology of Hebei province, College of Chemistry and Environmental Science, Hebei University, Baoding, 071002, China)
Received Aug. 21, 2002.
Abstract Based on the reversed phase ion-pair chromatography, the retention behaviour of thiourea, polysulfide, ammonium sulfide and ammonium thiocyanate were discussed under different conditions such as ion pair reagent and its concentration, ion intensity and organic reagent. It was testified that the concentration of ion pair reagent, pH and the volume ratio of tetramethylene-oxide in mobile phase played important roles in the retention behavior of ammonium sulfide and ammonium thiocyanate. The best analytical results were obtained by using a C18 column with a mobile phase comprising a 20:79:1 (% v/v) mixture of methanol-water-tetramethylene-oxide, pH 6.64, containing 3.0 mmolL-1 ammonium tetrabutyl bromide and 6 mmolL-1 KH2PO4-K2HPO4 buffer. Column temperature and detection wave were controlled at 25
ºC and 220nm, respectively. Linearity of the working curve was investigated with correlation coefficients of 0.9989-0.9999, and the relative standard deviation was below 4.9%. The proposed method had been successfully used in the samples determination.Keywords Reversed phase ion-pair chromatography, thiourea, polysulfide, ammonium sulfide, ammonium thiocyanate,retention behavior 1 INTRODUCTION
Ammonium thiocyanate and thiourea were two kinds of important chemicals and medicine materials[1,2]. And the conventional production process to obtain them were[3,4] :
CS2 + 2NH3 �� NH4SCN + H2S [1]
NH4SCN �� NH2CSNH2 [2]
The reaction [1] was often accompanied with a secondary reaction [3], which provided a by-product of polysulfied (NH4)2CS3. (NH4)2CS3 could be changed into ammonium thiocyanate described as equation [4] when heated[5].
2NH3 + H2S + CS2 �� (NH4)2CS3 [3]
(NH4)2CS3 �� NH4SCN + H2S [4]
In the preparation of ammonium thiocyanate, the mixture of ammonium thiocyanate, ammonium sulfide, and polysulfide was often gained, and when thiourea was prepared through isomerization of ammonium thiocyanate, the mixture of thiourea and ammonium thiocyanate was obtained. So the development of the qualitative and quantitative method for simultaneous determination of the components would provide a powerful means for their kinetics and mechanism study. In the previous work, Pertunina gave the titrimetric method for the detection of NH4SCN, (NH4)2S, NH3 and H2S step by step[6]. While Berestetskii reported a potentiometric titration of S2-, SCN- and S2O32- using a carbon indicator electrode[7]. NH2CSNH2 was usually detected by its UV absorption spectrometry[8]. But none of these methods could be directly used for the simultaneous determination of the four components. Reversed ion pair HPLC had been well used in the determination of organic acid, heterpolyacid, inorganic compounds and specific analysis[9-12]. But it had not been employed in the determination of NH4SCN, (NH4)2S, (NH4)2CS3 and NH2CSNH2 at the same time. In this paper, the reversed phase ion pair chromatography method was developed and used for the simultaneous and direct determination of the four components. The optimal separation condition was obtained through the study of retention behavior of the components under different conditions. The method had been successfully used in the samples detection.
2 EXPERIMENTAL
2.1 Instrument
A LC-6A HPLC was coupled to a SPD-6A ViS detector and C-R3A data processing system. The
Shim-Pack CLC C18 column (10mm, 150��6.0mm) was employed (Shimadzu, Japan). PHS-3C acidometer
and UV8500 Spectrophotometer were used (Shanghai).
2.2 Reagents
Ammonium sulfide was guarantee reagent (Tianjin, China). Polysulfide was prepared with carbon disulfide and ammonium sulfide according to the reference[13]. The yield of polysulfide was calculated according to the amount of carbon disulfide. All the reagents and solvents used were analytical grade.
3 RESULTS AND DISSCUSSION
3.1 The selection of ion pair reagent and its concentration
The UV absorption of the four components between 190 and 350nm was detected. To insure the
sensitivity, the analytical signal (peak area) was monitored at 220nm. In mobile phase,
ion pair reagent should have weak absorption at 220nm and have enough eletrostatic
attraction with the ion compounds. Four kinds of positive ion pair reagents such as benzyl
trimethyl ammonium chloride, cetyl trimethyl ammonium bromide, ammonium tetrabutyl iodide
and ammonium tetrabutyl bromide (TBA) were used, and TBA gave the higher sensitivity than
the others.
To study the influence of the concentration of TBA on retention
behavior, the fixed volume ratio of water, methanol and tetramethylene-oxide was used; the
ion intensity and pH were controlled by KH2PO4 �C K2HPO4 buffer solution. From Fig.1 we could
see that with the increasing of TBA concentration, the k' of NH2CSNH2 and (NH4)2CS3
had no obvious change, but the retention times of (NH4)2S and NH4SCN
were increased. So the concentration of ion pair reagent played an important role in the
separation of the four components.
3.2 Influence of pH on the retention behavior
The pH value of mobile phase had no effect on thiourea and polysulfide, but in order to keep ammonium of thiocyanic acid and hydrogen sulfide in ionic state, it should be controlled at above 6.0. With the increasing of pH from 6.10 to 7.17, the retention times of the ammonium thiocyanate and ammonium sulfide decreased, the results were shown as Fig. 2. This might be caused by the increasing of negative charge of phosphate, which promoted the complexing of phosphate with TBA, then decreased the complexing of TBA with thiocyanate and sulfide[11,14]. In the separation of the four components, pH played an important role.
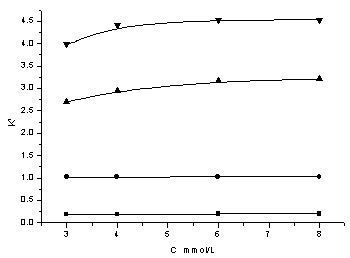 |
Fig. 1 Influence of
TBA concentration on retention ���� thiourea, ��polysulfide, ����ammonium sulfide,����ammonium thiocyanate |
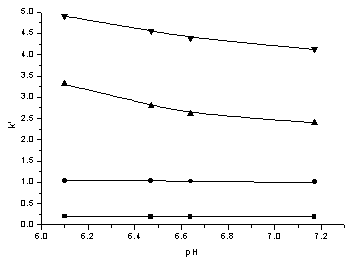 |
Fig. 2 Influence of
pH of mobile phase on retention ���� thiourea, ��polysulfide, ����ammonium sulfide,����ammonium thiocyanate |
3.3 The influence of organic reagents
on the retention behavior
When the volume ratio of methanol in water was increased, the retention times of the
components could be decreased. But the influence was weak in adjusting the retention
behavior of (NH4)2S and NH4SCN. Only when the value was
above 20%, could ammonium thiocyanate flow out from the column within 20min. With the
addition of 1% tetramethylene-oxide in mobile phase, the retention time of NH4SCN
could be decreased greatly (about 30%). This might be caused by the coordinated action of
strong dispersion force of tetramethylene-oxide and the increasing solubility of complexes
in mobile phase. So the content of tetramethylene-oxide played an important role in the
separation.
3.4 The influence of ion intensity on the retention behavior
When the ion intensity in mobile phase was improved, it has
weak influence on the retention times of NH2CSNH2 and (NH4)2CS3.
But the influence on (NH4)2S and NH4SCN were just the
same as that of TBA concentration.

Fig. 3 Chromatogram of four compositions
tR,I =3.113 min, tR,II =5.263 min, tR,III =9.795
min, tR,IV =14.263 min.
3.5 Optimization of the mobile phase
With the modification of the TBA concentration, pH, ion intensity, the volume ratio of water, methanol and tetramethylene-oxide, the optimal separation conditions were gained through uniform design experiment U7 (76) provided by Fang[15]. The mobile phase was 20:79:1 of methanol-water-tetramethylene-oxide, containing 3.0 mmolL-1 TBA and 6 mmolL-1 KH2PO4-K2HPO4 buffer solution with pH 6.64.
Under the proposed condition, the flow rate and column temperature were controlled at 1ml/min and 25ºC, respectively, the chromatogram of NH2CSNH2 (I), (NH4)2CS3 (II), (NH4)2S (III) and NH4SCN (IV) was obtained as Fig. 3.
3.6 The detection of linearity and recoveries
The relation between the peak area and the mass of the four components was examined. The linearity regression equations along with linearity ranges and correlation coefficients for determination of the four components are given in Table 1. The calibration curve was linear for the four components with correlation coefficients of 0.9989-0.9999.
A series of mixtures of the four components with different mole ratio, were determined, and the recoveries for the four components were examined. The results with relative standard deviation (RSD) were listed in Table 2. Recoveries and relative standard deviations were in the range of 94.0-106.0% and 0.76-4.9%, respectively, for the four components.
Table 1. The regression equation and linear scopes
Composition |
Regression equation |
linearity (mg) |
Correlation coefficient |
NH2CSNH2 |
Y=18665+228560x |
0.01905-18.27 |
0.9999 |
(NH4)2CS3 |
Y=34871+128660x |
0.03809-36.54 |
0.9995 |
(NH4)2S |
Y=-3249.7+105633.7x |
0.01703-35.81 |
0.9989 |
NH4SCN |
Y=77398+106675x |
0.07213-36.54 |
0.9992 |
Y: area of peak, X: the mass of composition (
mg) Table 2. Recoveries of NH2CSNH2 (I), (NH4)2CS3 (II), (NH4)2S (III), NH4SCN (IV)Composition |
I |
II |
III |
IV |
Recoveries |
98.6 �C100.7% | 101.3 �C102.9% |
94.0 �C97.3% |
98.0 �C106.0% |
RSD % (n=4) |
0.76 |
1.95 |
4.65 |
4.9 |
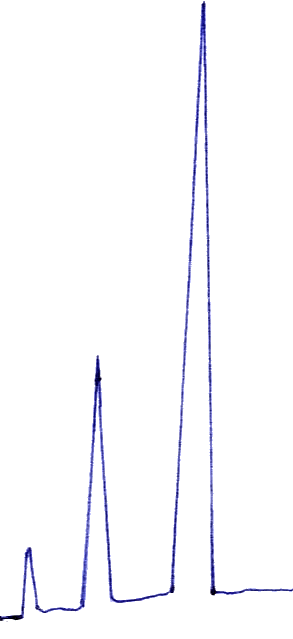
Fig. 4 The chromatogram of (NH4)2CS3, (NH4)2S and NH4SCN detected in synthesis mixture
��
Table 3. NH4SCN
isomerization temperature and the yield of NH2CSNH2
Temperature( ºC) |
100 |
120 |
127 |
140 |
150 |
170 |
Yield of NH2CSNH2 % |
6.32 |
14.50 |
17.95 |
34.21 |
27.93 |
25.67 |
3.7 Sample analysis
The method described above was used to the samples
analysis. The product which was gained through the reaction between carbon disulfide and
ammonia was analyzed, the chromatogram of (NH4)2CS3, (NH4)2S
and NH4SCN was detected as Fig.4. The isomerization products of NH4SCN
under different temperature were determined with the highest yield of thiourea obtained at
140ºC as listed in Table 3. The result was consistent with the
reference[5,16].
3.8 Conclusion
The proposed method was simple and easy with good accuracy and precision. It could
successfully be used for the simultaneous determination of ammonium thiocyanate synthesis
products and the isomerization mixture. The research provided a powerful means for the
kinetics and mechanism study.
REFERENCE
[1] Ga R D, Liu Y, Li G F et al. Spectroscopy and Spectral analysis (Guangpu yu
Guangpufenxi), 1997, 17 (5): 121.
[2] Sun L D, Liu C H, Liao C S et al. J. Mater. Chem (Cailiao Huaxue), 1999, 9 (8) : 1655.
[3] Pang X Y, Sun H W, Li Z Y et al. Chinese Journal of Applied Chemistry (Yingyong
Huaxue), 2002, 4, 407.
[4] Beleuskii S F, Sarukhanov M A, Kharitonov Y Y. Zh. Neorg. Khim, 1997, 42 (3): 502.
[5] Shinjiro K, Susumu F J. Chem. Soc. Japan, Ind. Chem. Sect, 1954, 57: 91.
[6] Petrunina A U, Kolchina N A. SU, 1,705,737, 1992
[7] Berestetskii U I, Tulgupa F M. Zh. Anal. Khim, 1989, 44 (2): 328.
[8] Zhou L Q, Cai H C, Li Y. Theory and Testing, Chemical Analysis (Lihua Jianyan), 1990,
26 (5): 293.
[9] Li H Y, Liang X M, Xue X Y. Chinese Journal of Analytical Chemistry (Fenxi Huaxue),
1995, 27 (5): 523.
[10] Wang Y P, Shen G Q, Zhu M H. Acta Chimica Sinica (Huaxue Xuebao),1993, 51: 386.
[11] Zhan X T, Yun Z H. Chinese Journal of Chromatography (Sepu), 1997, 15 (1): 57.
[12] Liang L N, Jiang G B, Hu J T. Chinese Journal of Analysis Laboratory (Fenxi
Shiyanshi), 2001, 20 (5).
[13] Bobbie D. S, Morris L. N. US, 2,893,835, 1959
[14] Malle A I, Gonzaez M J, Marina M L. Journal Chromato, 1992, 607: 207.
[15] Fang K T. Acta of Appl. Math(Yingyong Shuxue Xuebao), 1980, 3: 363.
[16] Chekhova C N. SU, 1,719,397, 1992.