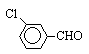http://www.chemistrymag.org/cji/2003/051008ne.htm |
Jan. 1, 2003 Vol.5 No.1 P.8 Copyright |
Zang Hongjun, Li Jitai#, Bian Yanjiang#,
Li Tongshuang#
(Research Institute of
Elemento-organic Chemistry, Key Labortary of Elemento-organic Chemistry, Nankai
University, Tianjin.300071; #College of Chemistry and Environmental Science, Hebei
University, Baoding 071002, China)
Received Sep.28, 2002. The project was supported by NSFC (29872011), Educational Ministry of China and Educational Department of Hebei Province; Natural Science Foundation of Hebei Province supported the project.
Abstract The coupling reaction of
aromatic aldehydes leading to pinacols were carried out by using Zn/montmorillonite
K10-ZnCl2 in 50% aqueous THF under ultrasound with 23-87% yield.
Keywords pinacolization, aromatic aldehyde, ultrasound
1 INTRODUCTION
1,2-Diols are very useful synthons for a variety of organic syntheses[1].
They can be used as intermediates for the preparation of ketones and alkenes. More
importantly, the pinacol coupling has been applied to the synthesis of biologically active
natural compounds[2].
The reductive coupling of carbonyl compounds is an important method for
the formation of 1,2-diols. A number of types of reagents such as Zn, TiCl3, Al
(Hg), Cp2TiCl[3], Ln[4], Mn[5,6], Sm-I2-MeOH[7],
Sm/TMSCl[8], Sm (Hg)[9], Te-KOH[10] and Al- KOH[11]
have been used to carry out the pinacol reaction. However, some of the used
reductants are expensive or the reduction conditions are critical. Previously, the
coupling of aromatic aldehydes and ketones had been reported to produce a-glycols using Zn-ZnCl2[12]
in low yield of pinacol product.
Ultrasound has increasingly been used in organic synthesis. Earlier
data clearly show that ultrasound irradiation can activate the surface of metal powder and
reduce the particle size, and bring about an effective surface modification[13].
Many metal-involved organic reactions have been accelerated under ultrasound. Recently, we
reported that ultrasonic irradiation could promote the pinacol coupling of aromatic
aldehydes by using Mg/NH4Cl[14].
Montmorillonite K-10 is known to behave as Bronsted acids in organic
reactions[15]. The use of K-10 as solid support has become very useful in
synthetic organic chemistry because of its enhanced selectivity due to its lamellar
swelling structure and large surface area. Up to now, the catalyst has been used as acidic
catalyst for many organic reactions. The advantages of the catalyst are easy handling,
chemical inertness, and lower cost, environmentally friendly and easy modification of
acidity by exchanging the cations in the interlayer space. In the meantime, organic
reactions in water or aqueous media have attracted increasing interest currently because
of the idea of green chemistry, environmental and economical issues, and understanding
biochemical process. All these prompt us to study the possibility for the pinacol coupling
using Zn/K10-ZnCl2 under ultrasound. In this paper, we wish to report the
results of the pinacol-coupling reaction of aromatic aldehydes and ketones in aqueous THF
under ultrasonication.
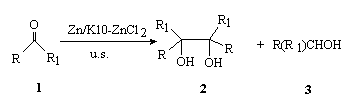
Scheme 1
2 EXPERIMENTAL
Liquid aldehydes were purified by distillation prior to use. IR spectra were recorded
on a Bio-Rad FTS-40 spectrometer (KBr). 1H NMR spectra were measured on
VXR-300S (300 MHz) using CDCl3 as solvent, TMS as internal standard. Mass
spectra were determined on an AEI MS-50 SD90 spectrometer (EI, 70 eV). Sonication was
performed in a Shanghai Branson-CQX ultrasonic cleaner with a frequency of 25 kHz and a
nominal power 500 W. The reaction flask was located at the maximum energy area in the
cleaner and the temperature of water bath was controlled by addition or removal of water.
General procedure: A 50ml Pyrex flask was charged with the desired
aldehyde or ketones (1, 1 mmol), Zn powder (0.4 g), K10-ZnCl2 (0.2 g),
and 50% aqueous THF (8 mL). The mixture was irradiated in the water bath of an ultrasonic
cleaner at the temperature for a period indicated in the Table 1. The reaction
mixture was combined with 3N HCl (1 mL) and filtered to remove the Zn powder. The filtrate
was extracted with toluene, washed with water, dried over anhydrous magnesium sulfate and
filtered. The toluene solution was evaporated under reduced pressure to give a mixture of 2
and 3. The mixture was separated by column chromatography on silica (200-300 mesh),
eluted with petroleum ether (b.p. 60-90 oC) or a mixture of petroleum ether and
diethyl ether. The authenticity of the product was established by their spectra.
2a: 1H NMR (CDCl3, 300 MHz): d 2.26 (s, 2H, OH), 4.70 (s, dl) and 4.83 (s, meso) (2H,
phCH-), 7.10-7.32 (m, 10H, Ar); MS: m/z (%), 214 (1), 180 (7.6),
167 (12.5), 149 (6.0), 107 (93.8), 79 (100), 77 (73.8); nmax (KBr): 3400-3480 cm-1
2b: 1H NMR (CDCl3, 300 MHz): d 2.98 (s, 2H, OH), 5.49 (s, meso) (2H, phCH-), 7.00-7.26
(m, 6H, Ar); MS: m/z (%), 318 (1.6), 316 (1.4), 179 (9.2), 177 (62.3), 176 (13.7),
175 (100), 147 (14.3), 111 (40.8), 77 (7.4); nmax (KBr): 3320-3400 cm-1
2c: 1H NMR (CDCl3, 300 MHz): d 2.98 (s, 2H, OH), 5.19 (s, dl) and 5.49 (s, meso) (2H,
phCH-), 7.10-7.26 (m, 8H, Ar); MS: m/z (%): 282 (10), 235 (12), 165 (5.5), 141
(100), 107 (19), 77 (10);nmax (KBr): 3380-3420 cm-1
2d: 1H NMR (CDCl3, 300 MHz): d 2.89 (s, 2H, OH), 4.62 (s, dl) and 4.79 (s, meso) (2H,
phCH-), 7.12-7.20 (m, 8H, Ar); MS: m/z (%), 263 (1.2), 251 (1.6), 178 (4.6), 165
(4.6), 141 (100), 113 (23.8), 77 (71.0); nmax (KBr): 3260-3318 cm-1
2e: 1H NMR (CDCl3, 300 MHz): d 1.79 (s, 2H, OH), 4.44 (8úČdl ) (2H, -CH-OH ), 6.29 (t, 2H, -CH=CH-), 6.71 (d, 2H, -CH=CH-),
7.24-7.41 (m, 10H, Ar); MS: m/z (%), 264 (1.0), 248 (6.2), 232 (3.6), 219
(3.0), 157 (6.7), 134 (21.4), 133 (100), 115 (24.7), 91 (27.8), 77 (19.9), 55 (37.0) nmax (KBr): 3300-3380 cm-1
2f: 1H NMR (CDCl3, 300 MHz): d2.90 (s, 2H, OH), 3.77 (s, dl) and 3.81
(s, meso) (s, 6H, OCH3), 4.64 (s, dl) and 4.75 (s, meso) (2H, phCH-), 6.73-7.21
(m, 8H, Ar); MS: m/z (%), 256 (1), 241 (1.2), 240 (5.2), 227 (10.8), 182 (5.7), 169
(3.0), 137 (100), 109 (26.1), 77 (24.9); nmax (KBr): 3300-3380 cm-1
2g: 1H NMR (CDCl3, 300 MHz): d 2.04 (s, 2H, -OH), 5.00 (s, dl) and 5.02 (s, meso) (2H,
furural-CH-), 6.27 (m, 4H, 3,4-furural-H), 7.26 (m, 2H, 5-furural-H) MS: m/z (%),
196 (10), 178 (25), 152 (73), 137 (33), 98 (100), 84 (22), 49(30); nmax (KBr): 3240-3300 cm-1
2h: 1H NMR (CDCl3, 300 MHz): d 1.26 (s, 2H, OH), 2.30 (s, dl) and 2.34 (s, meso) (6H,
CH3), 4.67 (s, dl) and 4.74 (s, meso) (2H, phCH-), 7.12-7.20 (m, 8H, Ar); MS:
m/z (%), 242 (1), 208 (2.1), 195 (3.8), 121 (100), 107 (20.4), 93 (43.8), 77 (20.5); nmax (KBr): 3280-3450 cm-1
2i: 1H NMR (CDCl3, 300 MHz): d 2.20 (s, 2H, OH), 4.57 (s, 2H, phCH-), 5.92 (s, 4H, CH2),
6.64-6.71 (m, 6H, Ar); MS: m/z (%), 302 (1), 284 (2.5), 268 (5.0), 255 (11.8), 151
(100), 123 (32), 93 (77.1), 65 (39.0); nmax (KBr): 3450-3490 cm-1
3 RESULTS AND DISCUSSION
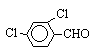
As shown in the Table 1 and the Scheme 1, the coupling of some aromatic
aldehydes gives pinacols in good yield in the presence of Zn/K10-ZnCl2 in THF
under ultrasonication. It is reported that the reaction proceeds via a single-electron
transfer mechanism with the Zn powder supplying the electrons. Ultrasonic irradiation
makes Zn active and increases the effective area. The data in the Table 1 show that
ultrasonic irradiation is a highly chemoselective method. For example, 1,2-di
(2,4-dichlorophenyl) -1,2-ethanediol and 1,2-dimethyl-1, 2-divinylbenzenyl- 1,2-ethanediol
(2b and 2e) were prepared in 87.1% and 74.8% yield respectively, using
Zn/K10-ZnCl2 under ultrasound. The effect of the substituents of the aromatic
ring on the yield is clear. The aromatic aldehydes with electron donating group show less
reactivity. In contrast, electron-withdrawing groups in the aromatic ring of aromatic
aldehydes increase the reactivity. The steric hinderance around the carbonyl group
inhibits the coupling reaction. When aromatic ketones such as acetophenone
and p-chloroacetophenone were used as
a substrate, no pinacol was obtained. The effect of the substituents of the aromatic ring
on the dl/meso ratio is not clear.
Table 1 Pinacol coupling of aromatic aldehydes and ketones using Zn/K10-ZnCl2 in aqueous THF under ultrasound
Entry |
Substrate |
t(h)/T(0C) |
dl/mesoa |
Isolated yield, %(lit) |
|
2 |
3 |
||||
a |
|
3/25-28 |
55:45 |
42.6(11)12 |
9.4(39)12 |
b |
|
2/22-24 |
meso |
87.1 |
13.6 |
c |
|
3/22-26 |
49:51 |
42.6(16)12 |
11.3(82)12 |
d |
|
3/28-30 |
51:49 |
73.6 |
18.7 |
e |
|
2/22-24 |
_ |
74.8 b |
20.4 |
f |
|
3/25-28 |
66:34 |
23.3 |
8.6 |
g |
|
3/25-28 |
66:34 |
61.2 |
1.8 |
h |
|
3/28-30 |
50:50 |
31.5(7)12 |
11(81)12 |
I |
|
3/25-28 |
_ |
33.1b |
21.5 |
j |
|
3/28-30 |
_ |
0 |
0 |
k |
|
3/28-30 |
_ |
0(0)12 |
0 |
a Ratios of dl/meso as calculated by 1H NMR.b The dl or meso was not determined. |
|||||
We also did the experiments in the absence of ultrasound, no pinacol was obtained when the substrate was benzaldehyde, and
the pinacol coupling product of 2,4-dichlorobenzaldehyde was obtained only in 28% yield
after stirring for 16h at r.t. Without the use of ZnCl2,
benzaldehyde and 2,4-dichlorobenzaldehyde were not coupled even irradiated for 8h at r.t
under ultrasound. The Zn/K10-ZnCl2 system is the best choice for the pinacol
coupling reaction under ultrasound. From a series of experiments, we found that the
K10-ZnCl2 could be reused under ultrasonic irradiation. For example, when the
substrate is the 2,4-dichlorobenzaldehyde (1b), the K10-ZnCl2 could be
cycled up to 3 times without substantial decreasing of the reactivity at the same
conditions, the yield of product is 87.1%, 86%, and 85% respectively.
In comparison with the classical method using Zn/ZnCl2[12],
the main advantage of the present procedure is milder reaction conditions, better chemical
selectivity, and the K10-ZnCl2 could be reused, thus, the environmental
concerns are also improved.
REFERENCES
[1] Nicolaou K C, Yang Z, Liu J J et al. Nature, 1994, 367: 630.
[2] Alexakis G A, Normant J F. Tetrahedron Lett., 1984: 3083.
[3] Wang L, Sun X H, Zhang Y M. J. Chem. Research (S), 1998: 336.
[4] Ding Z B, Wu S H. Chin. Org. Chem.(YouJi HuaXue), 1997, 17: 165.
[5] Rieke R D, Kim S H. J. Org. Chem., 1998, 63: 5235.
[6] Li C J, Meng Y, Yi X H, Ma J H. J. Org. Chem.,1998, 63: 7498
[7] Yanada R, Negoro N, Kanada K et al. Tetrahedron Lett.,1997, 38: 3271.
[8] Wang L, Zhang Y M. Tetrahedron, 1998, 54: 11129.
[9] Wang L, Zhang Y M. Synth. Commun., 1998, 28: 3991.
[10] Khan R H, Mathur R K, Ghosh A C. Synth. Commun., 1997, 27: 2193.
[11] Khurana J M, Sehgal A. J. Chem. Soc., Chem. Commun., 1994, 5: 571
[12] Tanaka K, Kishigami S, Toda F. J. Org. Chem., 1990, 55: 2981.
[13] Luche J L. Synthetic Organic Sonochemistry, Plenum Press, New York, 1998: 2.
[14] Li J T, Bian Y J, Liu S M et al. Synth.
Commun., 2002, 32: 547
[15] Laszlo P. Acc. Chem. Res., 1986, 19: 121