http://www.chemistrymag.org/cji/2003/052011pe.htm |
Jan. 1, 2003 Vol.5 No.2 P.11 Copyright |
(1Department of Chemistry, Jinan University, Guangzhou, 510632, China; 2Department of Biotechnology, Jinan University, Guangzhou, 510632, China; 3Department of Chemistry and Chemical Engineering, Division of Physical Sciences, Kanazawa University, 40-20, Kodatsuno 2-chome, Kanazawa, Ishikawa 920-8667, Japan)
Received Sep. 6, 2002.
Abstract Ternary liquid-liquid equilibria
for the acetic acid + triethylamine + chlorobenzene or toluene was measured at 293.15K.
The experimental results were compared with those calculated by the UNIQUAC
associated-solution model from the binary information. The model assumes that the
dimerization of the acid, 1:1, 2:1, 3:1 and 4:1 complex formation between the acid and the
amine, and non-specific interactions between unlike molecules present in the ternary
solutions. Good agreement between the experimental values and the results calculated by
the UNIQUAC associated-solution model was obtained.
Keywords Ternary liquid-liquid equilibrium, Associated mixtures, UNIQUAC
associated-solution model.
1. INTRODUCTION
Liquid mixtures of acetic acid with triethylamine exhibit phase separation at ambient
temperatures and have noticeably negative values of the excess Gibbs free energy. Kohler [1]
explained these phenomena by introducing complex formation between the acid and the amine,
but Kohler's model has not yet been developed to predict ternary liquid-liquid
equilibria(LLE) of mixtures containing acetic acid and triethylamine from binary
information alone. In this paper, we report the experimental LLE data for the ternary
mixtures composed of acetic acid, triethylamine with chlorobenzene or toluene at 293.15K,
and further apply the present model to reduce the LLE of the ternary mixtures from the
binary information.
2. EXPERIMENTS
Acetic acid, chlorobenzene and toluene were of guaranteed reagent grade (Wako Pure
Chemical Industries Ltd. of Japan, purity > 99.5 mass%). Triethylamine (purity >
99.5 mass%) was supplied by Fulka of Japan. The densities of all chemicals, measured with
an densimeter (Anton Paar, DMA 58) at 293.15K, were agreed well with literature. All
chemicals were used directly.
Ternary LLE measurements were carried out at (293.15\0.01)K. The
experimental apparatus are schematically shown in Figure 1. The mixtures were stirred by
using a magnetic stirrer for 3 hours, and settled for 4 hours. It is sufficient to
separate into two phases. The samples withdrawn from upper and lower phases were analyzed
by a gas chromatographic. The accuracy of the measurements was estimated within \0.001 in
mole fraction. Tables 1 and 2 show experimental LLE results for the two ternary systems of
acetic acid, triethylamine with chlorobenzene or toluene. 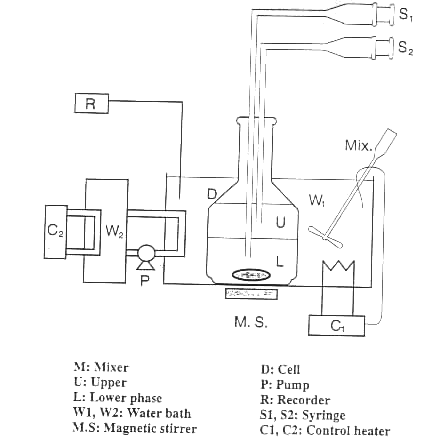
Fig. 1 Schematic diagram for liquid-liquid equilibria measurement
Table 1 Equilibrium phase compositions in
mole fraction (![]() ) for the ternary of
acetic acid(1) + toluene(2) + triethylamine(3) mixtures at 293.15 K
) for the ternary of
acetic acid(1) + toluene(2) + triethylamine(3) mixtures at 293.15 K
Upper phase |
Lower phase |
||||
x1 |
x2 |
x3 |
x1 |
x2 |
x3 |
0.1798 |
0.0000 |
0.8202 |
0.5824 |
0.0000 |
0.4176 |
0.1986 |
0.0310 |
0.7704 |
0.5480 |
0.0281 |
0.4239 |
0.2110 |
0.0488 |
0.7402 |
0.5280 |
0.0374 |
0.4346 |
0.2298 |
0.0712 |
0.6990 |
0.4975 |
0.0513 |
0.4512 |
0.2410 |
0.0802 |
0.6788 |
0.4865 |
0.0591 |
0.4544 |
Table 2 Equilibrium phase compositions in
mole fraction (![]() ) for the ternary
acetic acid(1) + chlorobenzene(2) + triethylamine(3) mixtures at 293.15 K
) for the ternary
acetic acid(1) + chlorobenzene(2) + triethylamine(3) mixtures at 293.15 K
Upper phase |
Lower phase |
||||
x1 |
x2 |
x3 |
x1 |
x2 |
x3 |
0.1798 |
0.0000 |
0.8202 |
0.5824 |
0.0000 |
0.4176 |
0.1962 |
0.0252 |
0.7786 |
0.5701 |
0.0102 |
0.4197 |
0.2076 |
0.0418 |
0.7506 |
0.5457 |
0.0237 |
0.4306 |
0.2144 |
0.0446 |
0.7410 |
0.5414 |
0.0269 |
0.4317 |
0.2403 |
0.0622 |
0.6975 |
0.5226 |
0.0398 |
0.4376 |
3. UNIQUAC ASSOCIATED-SOLUTION MODEL
Janet [2] proposed significant 1:1, 2:1, 3:1 and 4:1 complex formation between
the acid and the amine according to spectroscopic study. The
dimerization constant of A (acetic acid) is defined by
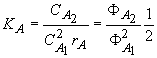 for A1 + A1 = A2
(1)
for A1 + A1 = A2
(1)
and the solvation constants for binary complexes between the acid and B (the amine) are
expressed as
![]() for A1
+ B1 = AB
(2)
for A1
+ B1 = AB
(2)
![]() for AB
+ A1 = A2B (3)
for AB
+ A1 = A2B (3)
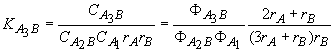 for A2B
+ A1 = A3B (4)
for A2B
+ A1 = A3B (4)
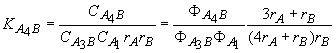 for A3B
+ A1 = A4B (5)
for A3B
+ A1 = A4B (5)
These equilibrium constants are characterized in terms of the segment
fractions and molecular volume parameters of chemical species present in the mixture. The
molecular structural parameters of the species are assumed to be expressed by those of
monomers: rA2 = 2rA, rAB = rA + rB,
rA2B = 2rA + rB, rA3B = 3rA + rB
and rA4B = 4rA + rB.
According to these chemical equilibria, the activity coefficient of
component i (i=A=acetic acid) in the ternary mixtures derived from
the model assumption is given by
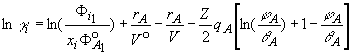
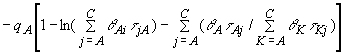 (6)
(6)
and the activity coefficients of non-associating component i (i=B,C;
B=triethylamine, C = chlorobenzene or toluene) are
expressed by
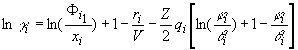
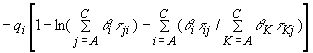 (7)
(7)
where the lattice coordination number Z is set to 10 and the segment fraction ![]() and the surface fraction
and the surface fraction ![]() are defined by
are defined by
![]() (8)
(8)
and the binary parameter ![]() is related to the binary energy parameter
is related to the binary energy parameter ![]() as:
as:
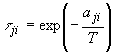 (9)
(9)
The true volume of the mixture V is given by
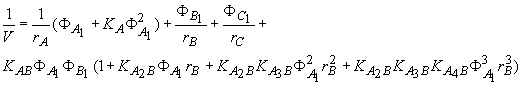 (10) and that of acetic acid in the pure state
(10) and that of acetic acid in the pure state ![]() is
is
![]() (11)
(11)
The monomeric segment fractions of components,![]() ,
, ![]() and
and ![]() , are obtained from simultaneous solution of the
following mass balance equations:
, are obtained from simultaneous solution of the
following mass balance equations:
![]()
![]() (12)
(12)
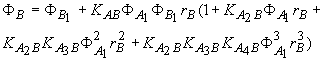 (13)
(13)
![]() (14)
(14)
The monomeric segment fraction in the pure acetic acid ![]() is expressed by
is expressed by
![]() =
[-1 + (8KA + 1) 0.5]/4KA
(15)
=
[-1 + (8KA + 1) 0.5]/4KA
(15)
4. CALCULATION PROCEDURE
The binary energy parameters of the model were obtained from the binary vapor-liquid
equilibria(VLE) data reduction using the following thermodynamic relation
![]() {
{![]() } (16)
} (16)
The fugacity coefficient of acetic acid was calculated by the chemical theory with gas
non-ideality[3]
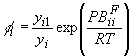 (17)
(17)
The fugacity coefficients of the vapor mixtures of the triethylamine, chlorobenzene and
toluene were calculated by
![]() (18)
(18)
where P is the total pressure, yi the vapor-phase mole fraction
of component i, yi1 the monomer mole fraction of component i in
the vapor phase, xi the liquid-phase mole fraction of component i,
and BiiF the free contribution to the second virial
coefficients of component i estimated by the method of Hayden and O'Connell [4].
The liquid molar volume is calculated from the modified Rackett equation [5].
The vapor pressures of pure components were calculated by the Antoine equation.
The binary energy parameters for the binary VLE data were obtained by
using a computer program described by Prausnitz et al. [6] and
minimizing the objective function:
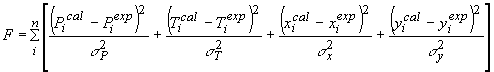 (19)
(19)
where the standard deviations in the measured valuables are taken as σP = 133.3 Pa in pressure,σT = 0.01<C in temperature,σx = 0.001 in liquid-phase mole fraction andσy = 0.003 in vapor-phase mole fraction.
LLE calculations were performed by solving the thermodynamic criteria
of equilibria that the activity of each component is the same in two liquid phases
and material balance.
![]() i
= A, B and C
(20)
i
= A, B and C
(20)
![]() and
and ![]() ( I, II = two liquid phases )
(21)
( I, II = two liquid phases )
(21)
In order to reproduce the number of adjustable parameters, we assumed KAB
= KA2B = KA3B = KA4B. After many trials, we adopted a
single value of 4.5〜103 for the four solvation constants at 293.15 K. The
association constants for acetic acid used in this work were taken from Tamura et al [7]:
KA = 79742.9 at 293.15 K. The pure-component molecular structural
parameters are estimated by the method of Vera et al. [8]
5. CALCULATED RESULTS
Table 3 presents the results of fitting the UNIQUAC associated-solution model to the
binary experimental values. The calculated results obtained from the UNIQUAC
associated-solution model involving association constant and solvation constant as well as
binary parameters alone were plotted in Figure 2. From the figure, we can see that
calculated results are in good agreement with experimental results for the ternary systems
determined in this work. It can be seen that in this work the assumption of the same four
solvation constants between the acid and the amine is reasonable. The performance ability
of the model for the associated solutions of the acid and the amine with aromatic
hydrocarbon was examined by comparing the calculated results with experimental tie-line
data.
Table 3 Calculated results of binary VLE and LLE data reduction
System (A+B) |
Temp./oC |
d P/kPa |
d T/K |
dx〜103 |
dy〜103 |
aAB/K |
aBA/K |
Ref. |
| Acetic acid + chlorobenzene |
40 |
0.02 |
0.00 |
0.0 |
8.6 |
54.25 |
21.64 |
(9) |
| Acetic acid + toluene |
30 |
0.03 |
0.00 |
0.0 |
5.8 |
56.45 |
32.67 |
(10) |
Acetic
acid + |
20 |
215.33 |
449.55 |
a |
||||
Triethylamine
+ |
70 |
0.11 |
0.01 |
2.9 |
7.1 |
226.42 |
-156.79 |
(11) |
Triethylamine
+ |
20 |
0.17 |
0.00 |
0.1 |
-107.68 |
161.79 |
(12) |
a: This work.
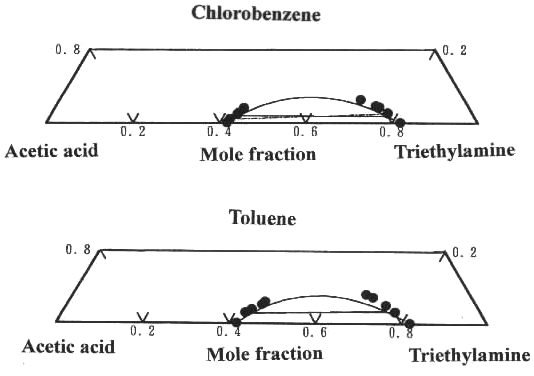
Fig. 2 Comparison between ternary experimental and calculated LLE of acetic acid + triethylamine + chlorobenzene or toluene at 293.15K. Experimental tie-line data: ¢-¢; Calculated by UNIQUAC associated-solution model: -.
6. CONCLUSION
The LLE results for the acetic acid, triethylamine and chlorobenzene or toluene measured
at 293.15K are presented. The experimental results were successfully reproduced from the
binary data by using the UNIQUAC associated-solution model taken into account the
demerization of the acetic acid and 1:1, 2:1, 3:1 and 4:1 complex formation between the
acetic acid and the triethylamine.
REFERENCES
[1] Kohler F, Bunsen Ber. Phys. Chem., 1978, 82: 582.
[2] Janet A, Tamada, Judson King C. Ind. Eng. Chem. Res., 1990, 29 (7): 1327.
[3] Nothnagel K H, Abrams D S, Prausnitz J M. Ind. Eng. Chem. Process Des. Dev., 1973, 12:
25.
[4] Hayden J G, O'Connell J P. Ind. Eng. Chem. Process Des. Dev., 1975, 14: 209.
[5] Spencer C F, Danner R P. J. Chem. Eng. Data, 1972, 17: 236.
[6] Prausnitz J M, Anderson T F, Grens E A, et al. Computer Calculations for
Multicomponent Vapor-Liquid and Liquid-Liquid Equilibria, Prentice-Hall, Englewood Cliffs,
New York, 1980.
[7] Tamura K, Nagata I. Fluid Phase Equilibria, 1991, 64: 49.
[8] Vera J H, Sayegh S G, Ratcliff G A. Fluid Phase Equilibria, 1977, 1: 113.
[9] Eoai E A, Cmpok H B, Ecaveuia E O et al. Russ. J. Phys. Chem., 1975, 41.
[10] Murkuzin N P, Pavlova L M. Zh. Prikl. Khim., 1971, 44: 311.
[11] Letcher T M, Anfd Bayles J W. J. Chem. Eng. Data, 1971, 16: 266.
[12] Kokonen P, Arvola H. Thermochimica Acta, 1984, 77: 333.