http://www.chemistrymag.org/cji/2003/052012ne.htm |
Jan. 1, 2003 Vol.5 No.2 P.12 Copyright |
( Department of Applied Chemistry, South China University of Technology, Guangzhou 510640, China)
Received Aug. 20, 2002; Supported by the National Natural Science Foundation of China ( Grant No. 29971011) and the Natural Science Foundation of Guangdong ( Grant No. 990653, 000489)
Abstract Success is achieved in the
microwave-induced intercalation of the larger guest molecule, chiral tailed porphyrin,
into an antimony hydrogen phosphate layered host material. The dielectric constant of
reaction medium was found to play an important role in the microwave-induced intercalation
synthesis and thus an organic acid solution with high dielectric constant was selected for
the intercalation system. The microwave inducement in the acid solution makes possible the
direct intercalation of protonated guest porphyrin into the layered host material. Our
experiments demonstrated an effective intercalation synthetic method for the preparation
of immobilized composite materials and better spacing channels are one of the advantages
of layered intercalated structure over guest molecule encapsulated zeolite structure.
Keywords Intercalation synthesis, microwave inducement, chiral tailed porphyrin,
layered materials
1 INTRODUCTION
One of the techniques for the preparation of advanced materials is intercalation synthesis
that incorporates or generates target guest molecules in the space available in pillared
lamellar structure [1-5] . These chemically synthesized composite materials
have been found with numerous applications, including size/shape/chiral selective
separation or purification, selective catalysis, artificial photosynthesis, long effective
drugs and so on. In our earlier work, we reported the in-situ intercalation [2-4] and
the microwave-induced intercalation [5] of tetraphenylporphyrin into layered
hosts to prepare layered host supported complexes. Recently we succeeded in the microwave
induced intercalation synthesis of chiral tailed porphyrin, 5[ p-( 1-L-phenylalanyloxy)
ethoxyl
2 EXPERIMENTAL
CTTPPH2 was prepared and characterized by our previously published method [6] . The product was purified by means of chromatography on silica gel with chloroform as an eluent. Final purple solid product CTTPPH2, as shown in Fig.1, was obtained with the yield of 55%.
Layered host material SbP was prepared by previously published method [1,2] , using solid state synthesis and then ionic exchanging. In an aqueous solution of acetic acid, SbP and required amount of chiral tailed porphyrin were stirred for about 30min at room temperature and then refluxed in MI-9700 microwave oven for the intercalation synthesis. The microwave frequency is 2450GHz. Crude product was then washed by water, ethanol and acetone respectively to exclude acetic acid solvent and surface absorbed porphyrin. The incorporation and crystalline structure of the tailed porphyrin in the intercalated materials depend on not only the molar ratio of SbP to chiral tailed porphyrin but also microwave inducement power and microwave inducement time. UV-Vis spectra were recorded with a DV-7( HS) spectrophotometer. The XRD data were recorded on a XD-3A X-ray diffractometer and element analyses were performed with a Perkin-Elmer 240 element analyzer.
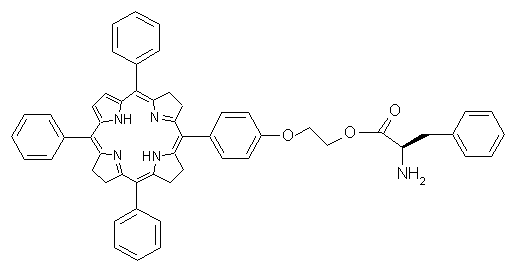
Fig.1 Chiral tailed porphyrin ( CTTPPH2)
3 RESULTS AND DISCUSSION
The molecular configuration of layered SbP is similar to the well-known layered
phosphatozirconic acid, ZrP, to a certain extent but the different metal atoms in their
structures result in obvious difference between their properties [1] . In SbP,
the metal, antimony, is in the
Our experiments showed that although the layered material SbP and porphyrin was refluxed for long time using various organic solvents such as acetone and methanol etc., porphyrin could not be intercalated directly into the interlamellar space of SbP, only a small amount of porphyrin was surface absorbed to the host. Using acetone as a reaction solvent, Cady [7] also failed in the direct intercalation of porphyrin into mica-type silicates. However, we found that in a proper concentration of aqueous acetic acid solution, the guest molecule, chiral tailed porphyrin CTTPPH2, even though it is so large a molecule, could be directly intercalated into antimony hydrogen phosphate layered host with the help of microwave inducement. Element analysis, X-ray diffraction, UV-Vis spectrum were used for the investigation of the intercalation synthesis.
A chloroform solution containing ( C2H5) 4N+ ion was used to partially exchange the guest chiral tailed porphyrin intercalated in the purified product and the UV-Vis spectrum of the solution displayed the typical bands of chiral tailed porphyrin at 419, 516, 551, 592 and 649 nm, as reported in our previous work [6] . It demonstrated the retention of the target guest molecule chiral tailed porphyrin CTTPPH2 within antimony hydrogen phosphate host. The elemental analysis of the purified solid product showed it could be incorporated up to 21.4% ( w: w) of chiral tailed porphyrin CTTPPH2 in SbP host, depending on the reaction conditions. Also, X-ray diffraction technique was used to monitor the process of intercalation synthesis. We found that the d002 spacing of SbP in connection with interlamellar distance was increased with the intercalation of the porphyrin. In a series of our experiments, we used the fixed microwave inducement power but adjusted microwave inducement time to control the intercalation reaction. The X-ray powder pattern exhibited the fine crystalline structure of the intercalated product and the increased d002 spacing of purified and dried porphyrin-intercalated product was 1.32 nm with a reflux intercalation reaction time of 30 min, as shown in the X-ray diffraction pattern ( a) of Fig.2, indicating a gallery height about 0.54 nm. In comparison with the thickness of porphyrin, the guest molecule should be somewhat slanted to the interlayer. Further uptake of chiral tail porphyrin under longer time of microwave inducement can result in a d002 spacing of 1.51 nm but the layered crystalline structure of host material SbP was found to become ruinous to a certain extent, shown in Fig.2, X-ray diffraction pattern ( b) .
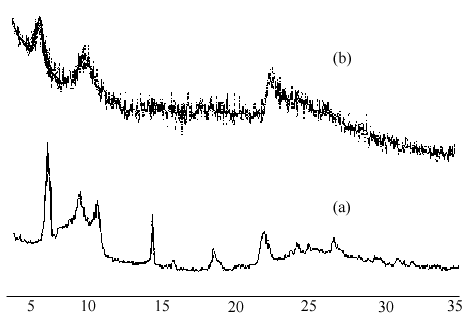
Fig.2 X-ray diffraction patterns of the intercalated product:( a) 30 min reflux time; ( b) 4 hrs reflux time
The overall reactions
in the intercalation can be written as the following:
![]()
![]()
In our earlier research we found that the microwave inducement was in
direct proportion to dielectric constant of reaction medium [8] . In this
microwave induced reaction system, acetic acid is a kind of reaction medium that possesses
higher dielectric constant in aqueous solution than acetone or methanol as the reaction
medium. On the other hand£¬the tailed porphyrin can
form a kind of protonated ions in the aqueous solution of acetic acid£¬the protonated form of porphyrin is much easier than porphyrin
itself to be microwave-induced and be intercalated into the layered phosphate host to
replace the active hydrogen ion in the interlamellar space of SbP host. Layered
intercalated structures are usually more easily fine tuned than 3 dimensional fixed
structures such as zeolites. One of the advantages of layered intercalated structure of
the chiral porphyrin over zeolite-encapsulated structure for the preparation of composite
materials is that layered intercalated structure can provide better spacing channel for
reaction components or eluates to diffuse in it.
In our experiments, we found the microwave induced intercalation
synthesis is not only to give rise to good reproducibility but also better to control the
chiral porphyrin content in layered phosphate host material than our previous in-situ
synthesis of porphyrin. More important, it provides an effective intercalation synthetic
method for some larger molecule encapsulated composite materials.
REFERENCES
[1] Hudson M J, Locke William, Mitchell P C H et al. Solid State lonics, 1993, 61: 131.
[2] Hu Ximing, Huang Zhongtao, Gu Guobang et al. J. Mol. Catal., A: Chem, 1998, 132: 171.
[3] Hu Ximing, Chen Biyun, Pi Zongxin, et al. Chemical Journal of Chinese University
(Gaodeng Xuexiao Huaxue Xuebao), 1997, 18 (2) : 107.
[4] Hu Ximing, Pi Zongxin, Zhongtao Huang et al. Chemical Journal of Chinese University
(Gaodeng Xuexiao Huaxue Xuebao), 1996, 17 (9) : 1353.
[5] Hu Ximing, Huang Jian, Liu Haiyang et al. Journal of Molecular Catalysis (Fenzi
Cuihua), 1998, 12 (2): 87.
[6] Liu Haiyang, Hu Ximing, Liu Yi et al. Molecules, 1999,
[7] Cady S S, Pinnavaia T J. Inorg. Chem., 1978, 17: 1501.
[8] Zhang Hualian, Hu Ximing, Lai Shengli. Journal of South China University of Technology (Huanan Ligong Daxue Xuebao), 1997, 25 (9): 46-50. ¡¡