http://www.chemistrymag.org/cji/2003/052013pe.htm |
Jan. 1, 2003 Vol.5 No.2 P.13 Copyright |
Preparation, characterization and properties of Keggin-type heteropolyanion Langmuir-Blodgett film
Zhong Lifeng, Tang Yu, Zhong Jiuping, Wang
Xiaoyan,Ouyang Jianming, Zhang Yuanming*
(Department of Chemistry, Ji'nan University, Guangzhou 510632, China)
Supported by the National Natural Science Foundation of China (No.29903004).
Abstract The possibility of the formation
of Langmuir-Blodgett(LB) film with octadecylamine hydrochloride(ODA) after the addition of
divanadium(V)-substituted
Keggin-type molybdophosphate anion PMo10V2O405- into
the subphase has been explored. Remarkable modifications of the compression isotherms are
observed when this heteropolyanion is dissolved in the subphase, which indicates that the
heteropolyanion interact with the ODA monolayer. LB film has been readily obtained from
this system. The atomic force microscopy (AFM), low-angle X-ray diffration(LAXD), Fourier
transform IR(FT-IR) spectroscopy and ultra violet (UV) spectroscopy have been used to
investigate the morphology and molecular structure of the deposited ODA/ PMo10V2O405-
LB film. AFM images have shown that the ODA/ PMo10V2O405-
LB film is relatively homogeneous and without domain structure even after a long time.
X-ray diffraction data have revealed that the ODA/ PMo10V2O405-
LB film possesses of lamellar structure and defines well among the layers. The calculated
results indicate that the chain of ODA molecule is almost vertical to the plane of quartz
slide. The characteristic adsorption peaks of heteropolyanion are not observed in the IR
spectra. But there are asymmetrically and symmetrically stretch vibration of long chain
alkyl. This demonstrates that ODA has deposited on the quartz slide successfully. UV
spectra results indicate that the PMo10V2O405-
ion has arranged by dense packing and oriented orderly in film. All the experiments
carried out in this study suggest that the new materials of heteropolyanions can be formed
by LB techniques and beneficial physico-chemical properties of heteropolyanions can be
maintained or enhanced through molecular-level design.
Keywords Langmuir-Blodgett film, Heteropolyanion, Low-angle
X-ray diffration; Atomic force microscopy, Fourier transform infrared spectroscopy.
Interest in transition-metal-substituted heteropolyanion is growing in the fields of catalysis, material science, biology and medicine because of their chemical, structural and electronic versatility[1-6].Especially in the field of electrocatalysis, owing to the ability of heteropolyanion to accept various numbers of electrons giving rise to mixed-valency species(heteropolyblue and heteropolyblown), significant research efforts have been directed towards the attachment of heteropolyanions on electrode surfaces with the main aim to prepare a new type of catalyst for various applications in heterogeneous electrocatalysis[7-9].Anson et al.[8] had been successfully immobilized metal-substituted heteropolyanions in polypyrrole but the modified electrode seemed to be degraded easily and the anion was lost. It was reported[10] that the modified electrode obtained from poly(N-methylpyrrole) reveals electrochemical stability and catalytic activity for the reduction of nitrite. However, the redox sites were random in the film. To choose a reasonable attachment method for improving the ordering and properties of such multilayer film, the Langmuir-Blodgett(LB) technique may be a useful tool.
LB technique can fabricate film with a large-scale ordering and can also provide new insight into electron transfer reactions at the interface[11].So using the LB technique to immobilize transition-metal-substituted heteropolyanion on self assembled monolayer should control their properties better. However, the reports concerning to the experimental methods of fabricating polyoxometalate LB film based on an ionic association between a heteropolyanion and a long-chain fatty acid or quaternary ammonium or similar molecule are rather scarce. Clemente-Leon et al.[12] showed that the semiamphiphilic approach seems to be the easiest way to obtain such organized molecular assemblies. Using this method, S. Liu et al.[13] reported an immobilization method of transition-metal-sbustituted heteropolyanion where transition- metal-substituted heteropolyanion was associated with amphiphilic cations. In this work, the divanadium(V)-substituted molybdophosphate anion with Keggin-type, PMo10V2O405-,and the primary amine with positive charge which was used as depositing film, octadecylamine hydrochloride(ODA) have been selected. Using the method of vertical lifting, we get the Y-type LB film containing such heteropolyanion, and then characterize them by AFM, LAXD. adsorption FT-IR spectroscopy and adsorption UV spectroscopy. These results indicate that by using this method ultrathin film with regularity in molecular organization can be obtained. 2. EXPERIMENTAL SECTION
2.1 Chemicals and instruments
Vanadium(V)-substituted Keggin-type molybdophosphoric acid,H5PMo10V2O40 13H2O (abbreviated PMo10V2) was synthesized based on literature method[14,15]. The structural formula was understood by the analysis of TG and ICP-AES. The results of FT-IR spectrum and XRD show that this compound has Keggin structure with A-type. octadecylamine hydrochloride(ODA) was from Alfa Aesar. Chloroform was used as spreading solvent and was redistilled before use. The GC result of this chloroform display that there is unique tip peak. All other chemicals were of analytical grade and were used as received. The water for preparing solution was gotten from a SYZ-550 quartz subboiling super pure distillatory and was distilled double time before using.
Measurement of surface pressure-area(p-A) isotherms was carried out with a WM-2 Langmuir trough system, which is a fully computerized and programmable apparatus fitted with two movable Teflon barriers. AFM images were obtained with an Autoprobe CP Research model from THERMO MICROSCOPE(USA) instruments with a APSC-0100 model scanner. Commercially microfabricated Si3N4 cantilevers with integrated pyramidal tips(180mm) and a spring constant of 3.2 N/m were used. Tapping imaging model was used. All images were recorded in 512×512 pixels and treated by flatten auto. FT-IR spectra were recorded in Bruker EQUINOX 55 spectrometer. LAXD experiments were performed with a D/max-3A model X-ray powder diffractometer(Rigaku Corporation). UV spectra were obtained with an UV-2501 PC UV-VIS Recording Spectrophotometer(Shimadzu Corporation).
2.2 Preparation of LB film
The measurements of surface pressure-area isotherm and the deposition of LB film were carried out at 25±1ºC. The monolayer of the ODA was formed by spreading out a ca.1.0×10-3 mol/L ODA chloroform solution onto a 1.0×10-5 mol/L H5PMo10V2O40·13H2O aqueous solution(subphase) whose pH is equal to ca.5.1. The compression was performed using a constant speed for the barrier of ca.10 mm/min. Assembled Y-type film has been obtained by the vertical lifting mode method with the lifting speed of 2 mm/min. The monolayer was transferred onto a quartz substrates which was treated with dichlorodimethylsilane at a constant surface pressure of 20mN/m. All the LB films are composed of twelve layers. The obtained LB films were placed vertically in a beaker and isolated air by seal film. The following series of characterizations indicate the transfer ratio is up to about 0.95 on lifting film using surface pressure of 20mN/m, and the multilayer is stable well. 3. RESULTS AND DISCUSSION
3.1 Monolayers of ODA at the air-water interface
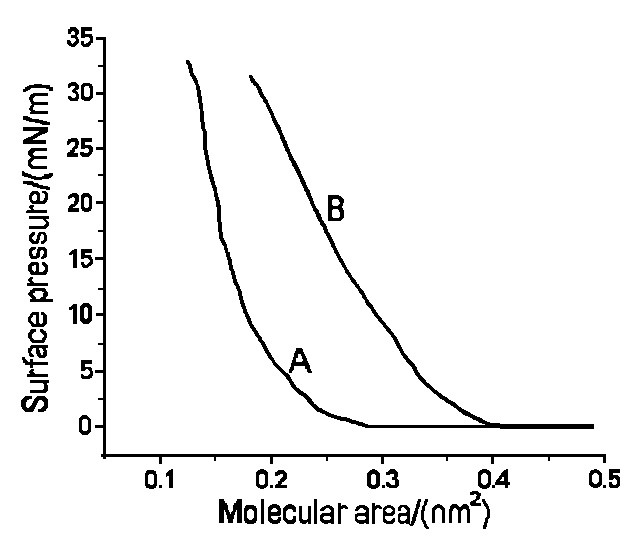
Fig. 1 Compression isotherm of ODA on pure water(A)and on a
10-5M PMo10V2 solution(B)
The surface pressure-area isotherms(p-A curves) for
ODA on pure water(curve A) and on a 1.0×10-5 mol/L H5PMo10V2O40
13H2O aqueous solution(curve B) are presented in Fig. 1. ODA can form stable
monolayer not only at the air-water interface but also at the air-aqueous solution of
heteropolyanion interface. In Fig. 1, curve A and curve B all have air-phase, liquid-phase
and solid-phase segment. The collapse pressure of monolayer of curve A and curve B are
about 33mN/m and 32mN/m respectively. Drawing the tangent of solid-phase curve, we obtain
that the area per molecule are ca. 0.31nm2 of curve B and 0.19nm2
of curve A. When a PMo10V2 anion is added to the pure
water subphase, the compression isotherm of ODA is progressively modified, as shown in
Fig. 1B. The isotherm is shifted towards bigger area per molecule and at the same time,
the isotherm shows a slower rise. We know the cross-section area per ODA molecule is about
0.19nm2. But the radium of PMo10V2O405-
heteropolyanion is ca. 0.5nm. When a PMo10V2O405-
anion is added to the subphase, the monolayer of ODA will attach a layer of
heteropolyanion by electrostatic interaction, markedly which results in bigger
cross-section area. On the other hand, the PMo10V2O405-
heteropolyanion possesses of five negative charge. Accompanying by the surface pressure
increase, the strong ionic repulsive interaction brings on a slower rise of isotherm.
3.2 Morphological observation of ODA/ PMo10V2O405-
LB film
AFM has been used as an important tool for studying LB film recently[16,17]
because of its all-time ability to give information ranging from molecular resolution
crystallography to surface morphology at length scales up to about 100μm on film with any number layers. So AFM studies of ODA/ PMo10V2O405-
LB film may be an ideal method for observing the surface morphology and defects in LB
film. Parts A, B in Fig. 2 display the typically planar and three-dimensional images of
ODA/ PMo10V2O405- LB film deposited on quartz
substrates. Part C in Fig. 2 shows the cross-section of Fig. 2A,B. That the particles of
heteropoly acid distribute on the ODA monolayer homogeneously was observed from Parts A, B
in Fig. 2. The particles' size is ca. 50nm and the
height fasten primarily on 7-8nm. The homogeneity of the film suggests that the molecules
deposited on the substrate are of relatively dense packing, which is similar to the
transfer of a conventional amphiphilic materials such as fatty acids. It is very important
to build ODA/ PMo10V2O405- LB film
supermolecular assemblies with well-defined molecular arrangement and orientation. The
average roughness(Ra), the RMS roughness(Rq), the peak height(Rp)
and the valley height(Rv) are equal to 4.247, 1.754, 3.531, -3.094nm
respectively from Part C. On the other hand, the average roughness of 4.247nm also
demonstrates that even for twelve-layer LB film of ODA/ PMo10V2, an
extremely regular surface morphology was observed over the large scale(1×1mm) and did not display any evidence for
existence of domain structures.

Fig. 2 AFM images of ODA/ PMo10V2O405-
LB film on quartz
slide: (A) ichnography, (B) three-dimension, (C) cross-section.
3.3 Structural characterization of ODA/
PMo10V2O405- LB film
3.3.1 Low-angle X-ray diffraction
The LAXD pattern of ODA/ PMo10V2O405- LB
film deposited on the quartz slide in Fig.3 is presented, which is composed of twelve
layer. Evidently, there are five peaks and the corresponding value of 2
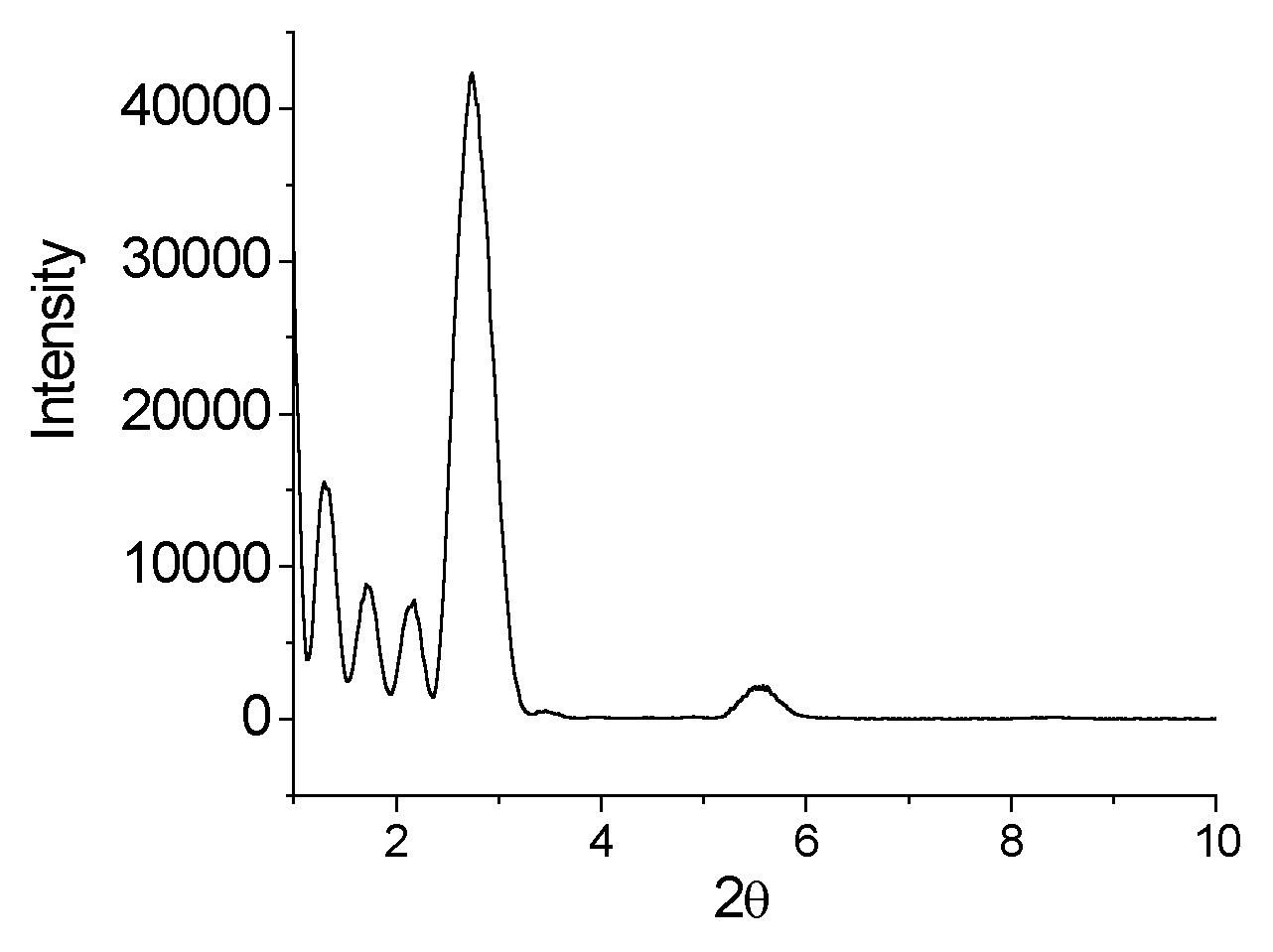
Fig.3 LAXD pattern of ODA/PMoV2 LB film
According to the equation of Bragg (nl =2dsinq), we have calculated the d value equal to 6.79 nm. To Y-type film, the d/2 is just the thickness of monolayer, i.e. the thickness of monolayer is equal to ca. 3.4 nm. We know that the length of ODA is ca.2.4 nm and the radius of this heteropolyanion is ca. 0.5 nm. So we draw some conclusions. (1) There is one layer heteropolyanions in the middle of ODA lamellar structure in the LB film. (2) These heteropolyanions are separated by ODA molecule. (3) The chain of ODA molecule is almost vertical to the plane of quartz slide.
3.3.2. FT IR spectra
The FT IR spectra of ODA/ PMo10V2O405- LB film was determined. From the pattern, two middle strength adsorption peaks lying in 2916.91 and 2849.06 cm-1 were observed. Judged from these, there exist asymmetrically and symmetrically stretch vibration of C-H in long chain alkyl. These indicate that ODA monolayer was deposited onto the quartz slide successfully. However, because the quartz slide can almost fully adsorb the radiation below the 2065 cm-1, the four characteristic adsorption peaks of heteropolyanion from 1100 cm-1-700 cm-1 were covered completely. So we cannot observe the characteristic adsorption peaks of heteropolyanion from the FT IR spectra pattern.
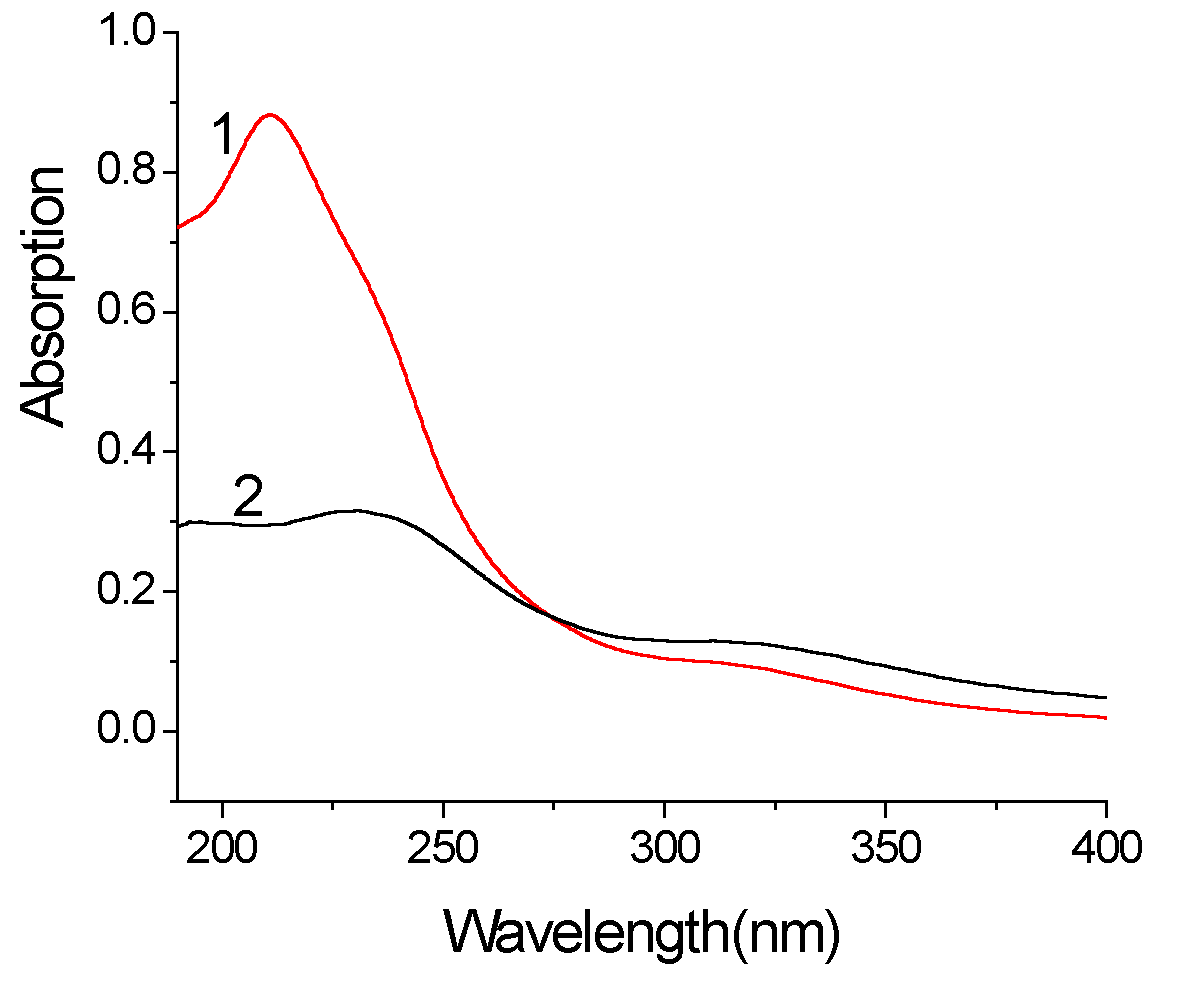
3.3.3 UV spectra
There is prominent significance to study the electronic spectra of ODA/ PMo10V2O405- LB film for understanding the electron transfer and energy relation between molecules. The UV spectra of twelve-layer ODA/ PMo10V2O405- LB film(curve 2) and the parent solution(curve 1) are presented in Fig. 4. Compared curve 1 with curve 2, obviously, the absorption strength of parent solution is larger than that of ODA/ PMo10V2O405- LB film. Both of them have two absorption peaks, whose value are 210.80, 303.20nm of curve 1 and 230.20, 311.00nm of curve 2 respectively and the former absorption strength is greater than the latter. We know that the former corresponds to the charge-transfer transition spectrum of Od→M and the latter corresponds to the charge-transfer transition spectrum of Ob(c) → M. All the absorption peaks of ODA/ PMo10V2O405- LB film show red-transfer compared with those of parent solution. The former shifts 19.4nm and the later shifts 7.8nm. These indicate that the PMo10V2O405- ion has arranged by dense packing and oriented orderly in film. The main reason is that there exists some electrostatic interaction between heteropolyanion and hydrophobic group which reduces the energy of charge-transfer transition. 4. CONCLUSION
This study has demonstrated that the adsorption properties of vanadium(V)-substituted molybdophosphate along ODA monolayers could be used to form the organized molecular assemblies. When the PMo10V2O405- anion is added to the subphase, the compression isotherm of ODA is shifted towards bigger cross-section area per molecule and shows a slower rise due to the ionic repulsive interaction. AFM images have shown that the ODA/ PMo10V2O405- LB film are relatively homogeneous and without domain structure even after a long time. Low-angle X-ray diffraction data have revealed that the ODA/ PMo10V2O405- LB film possesses of lamellar structure and defines well among layers. The calculated results indicate the chain of ODA molecule is almost vertical to the plane of quartz substrate. The characteristic adsorption peaks of heteropolyanion are not observed in the FT IR spectra. But there are asymmetrically and symmetrically stretch vibration of C-H in long chain alkyl. These demonstrate that ODA has deposited on the quartz slide successfully. UV spectra results have shown that the absorption peaks of ODA/ PMo10V2O405- LB film take place red-transfer compared with parent solution. This indicates that the PMo10V2O405- ion arrange by dense packing and orient orderly in film. The obtained ODA/ PMo10V2O405- LB film is stable.
REFERENCES
[1] Lyon D K, Miller W K, Novet T et al. J. Am. Chem. Soc., 1991, 113: 7209.
[2] Katsoulis D E, Pope M T, J. Am. Chem. Soc., 1984, 106: 2737.
[3] Mizuno N, Misono M. J. Mol. Catal., 1994, 86: 319.
[4] Pope M T. Heteropoly and Isopoly Oxometalates, Berlin: Springer-Verlag, 1983.
[5] Pope M T, Muller A. Polyoxometalates: from Platonic Solids to Antiretrovival Activity, Dordrecht: Kluwer, 1994.
[6] Misono M. Catal. Rev. Sci. Eng., 1987, 29: 269.
[7] Toth J E, Anson F C. J. Electroanal. Chem., 1988, 256: 361.
[8] Shin K, Anson F C. J. Electroanal. Chem., 1991, 309: 115.
[9] Dong S, Liu M. J. Electroanal. Chem., 1994, 372: 95.
[10] Fabre B, Bidan G, Lapkowski M. J. Chem. Soc. Chem. Commun.,1994:1509
[11] Goldenberg L M. J. Electroanal. Chem., 1994, 379: 3.
[12] Clemente-Leon M, Agricole B, Mingotand C et al. Langmuir, 1997, 13: 2340.
[13] LiU S, Tang Z, Wang E, Dong S. Thin Solid Film, 1999, 339: 277.
[14] Tsigdinos G A, Hallada C J. Inorg. Chem., 1968, 7 (3): 437.
[15] Buecheler N M, Sythesis and characterization of heteropoly acids and related materials[PHD],The University of Temple,1997.
[16] Garnaes J, Schwartz D K, Viswanathan R et al. Nature, 1992, 357: 54.
[17] Schwartz D K, Garnaes J, Viswanathan R et al. Science, 1992, 257: 508.
[18] He X Z, Zhou Y L, Wang L X et al. Science in China (ZhongGuo Kexue B), 1998, 14 (6): 633.