http://www.chemistrymag.org/cji/2003/052017pe.htm |
Jan. 1, 2003 Vol.5 No.2 P.17 Copyright |
A study on fluorescence spectra of Schiff bases
Yang Huisen, Xia Xinfu
#( Department of Pharmacy, Shaanxi College of Traditional Chinese medicine, Xianyang, 712083; #Department of Chemistry , University Shihezi, Shihezi, 832003)
Received Sep. 9, 2002.
Abstract Schiff bases with the typical
structure were synthesized, their fluorescence spectra were determined in solid state, and
photochromism and thermochromism of the related Schiff bases were investigated. The result
shows that mono-Shiff bases have symmetrical excitation and emission spectra, while the
excitation and emission spectra are asymmetric for bis-Schiff bases. No photochromism is
found with mono-Schiff bases, while both photochromism and thermochromism are obvious for
bis-Schiff bases.
Keywords Schiff base, fluorescence spectrum, photochromism, thermochromism
In view of the fact that information will be transmitted and processed on the optical medium , the development of compounds used for storing of information, optical driving, non-linear optical appliances and others has emphasized significance [1-3] . Schiff bases have photochromic and thermochromic properties, many of them have non-linear optical property, and all of these have been given great attention now. This paper reports fluorescence spectra of the Schiff bases with typical structure, and preliminarily studies on photochromism and thermochromism of the related Schiff bases. These results will be helpful for probing into the relationship between their spectra and structures.
1 EXPERIMENTAL1.1 Instruments and materials
Salicylaldehyde( A.R), benzaldehyde( A.R), aniline( A.R), diethylenetriamine( A.R), o-Phenylenediamine ( A.R), p-Phenylenediamine( A.R) absolute alcohol( A.R).
LS50B fluorescence /luminescence /phosphorescence spectrograph, ultraviolet lamp.
1.2 Synthesis of Schiff bases with different structures
An aldehyde of certain amount was added with 100ml of absolute alcohol in a three-necked round bottom flask, after the content was shake up, an amine of the calculated amount as the reactant proportion was added. The reaction mixture was stirred until yellow or jacinth crystals precipitated out. If no crystal formed, the reaction mixture was concentrated to remove the alcohol until the crystal precipitated. The powdered was filtered, washed and then recrystallized in alcohol. Finally, the obtained powdered was placed at a dark vessel. Take different aldehydes and amines and repeat the above-said procedure to synthesize other Schiff bases, the obtained compounds are identified as follows:
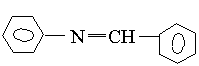
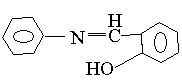
1 2
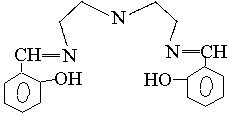
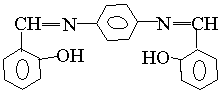
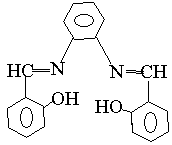
3 4 5
The elements analysis
shows( the theory value in the bracket):
Compound 1, C:85.83%(86.19%), H: 6.01%(6.08%), N:7.69%(7.73%);
Compound 2, C:79.01%(79.29%), H: 5.46%(5.53%), N:8.81%(8.93%);
Compound 3, C:76.70%(76.92%),H:5.21%(5.13%), N:9.29%(8.97%);
Compound 4, C:76.68%(76.92%),H:4.91%(5.13%), N:9.20%(8.97%);
Compound 5, C:73.41%( 73.72%), H:6.80%( 7.17%), N:9.72%(9.56%).
These compounds all have characteristic absorption of C=N at 1610cm-1
in their IR spectra.
1.3 Spectral properties of the Schiff bases
Excitation and emission spectra of the synthesized Schiff bases with different structures
were detected in LS50B fluorescence/luminescence/phosphorescence spectrometers .Relevant
results showed in Figures 1-5.
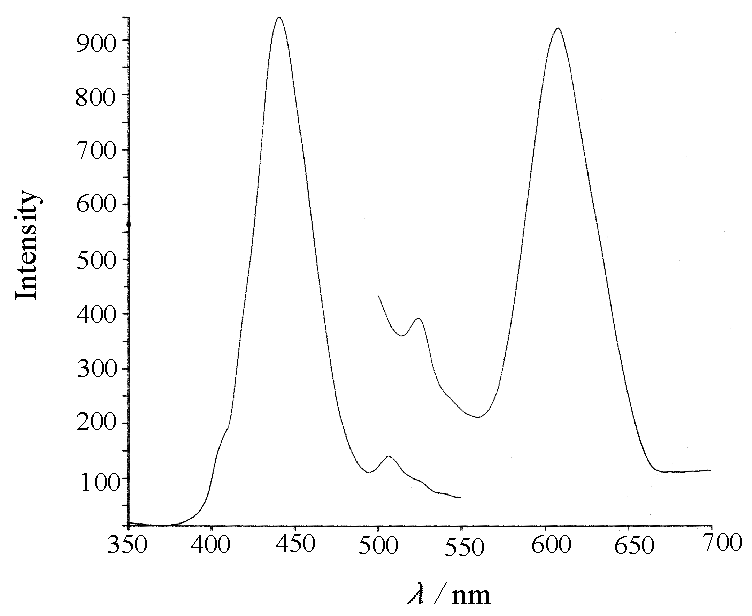
Fig.1 The excitation
and emission spectra of benzalaniline
l
2 RESULTS AND DISCUSSION
2.1 Relationship between the colors and structures of the synthesized products
There are two categories in the synthesized Schiff
bases, one is the Schiff base poly condensed from salicylaldehyde and different amines,
another is the Schiff base from benzaldehyde and different amines. A rule may be found
with the both categories, i.e., the color of the former is darker than that of the latter.
This is because there is a hydroxyl group on which an atom with unpaired electrons is as
the auxochromic group in the molecule structure of salicylaldehyde. When the atom is
introduced into a conjugated system, the unpaired electrons on the hydroxyl group also
enter the conjugated system, the delocalization of p-electrons
in the molecule is enhanced, resulting in darker color of the compound.
2.2 Characteristics of fluorescence spectrum
of the Schiff bases
Excitation and emission spectra of some synthesized
Schiff bases are illustrated in Figures 1 - 5 respectively. And the detection of
fluorescence spectra condition is reflected in the solid state of front surface.
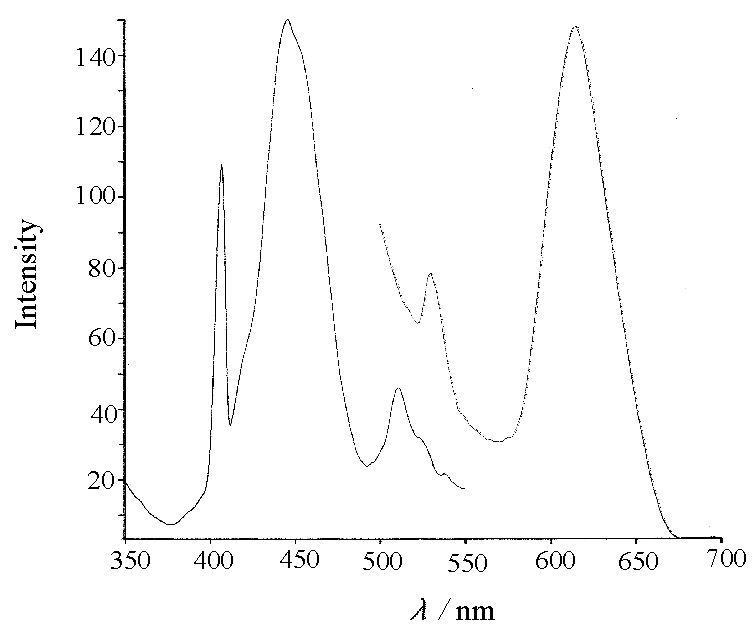
Fig. 2 The excitation and emission
spectra of salicylanilin
nm lex= 450 nm lem= 624 nm
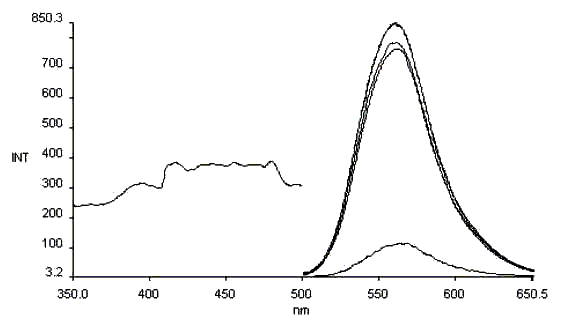
Fig. 3 The excitation and emission spectra of salicyl-o-diaminobenzene
lex=392, 414, 477nm lem = 560nm
It may be seen from Figs.1 through 5
that both the excitation and emission spectra of mono-Schiff bases have more stronger
symmetry. While the excitation spectra of bis-Schiff bases are more complex, their
emission spectra are relatively simple, and both the spectra have more stronger asymmetry.
For the bis-Schiff bases, only emission spectra with the same wavelength may be obtained
regardless of different excitation wavelengths, and their spectra, no matter excitation or
emission spectrum, have relatively less symmetry due to difficulty to conjugate, on the
other hand, the excitation and emission spectra are more symmetrical for the mono-Schiff
bases, which structures are readily subjected to conjugation.
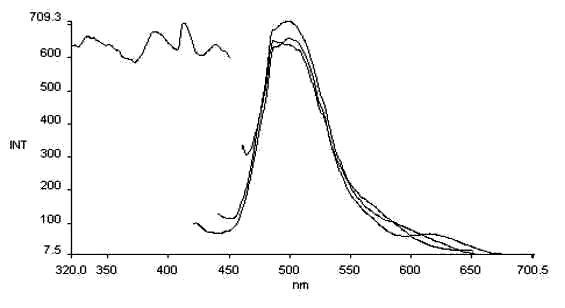
Fig .4 The excitation and
emission spectra of salicyl-p-diaminobenzene
lex= 370nm
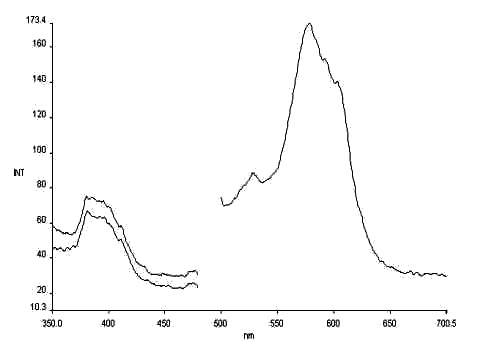
lex= 385,412, 440nm lem= 576nm
2.3 Photochromism
It is found in the detection of excitation spectra of Schiff bases after expose them to
ultraviolet lamp for 1min each time that very obvious photochromism appears for
salicylaniline(Fig 6), salicyl-o-diaminobenzene(Fig7), or salicyl diehylenetriamine (Fig
8) in solid state.
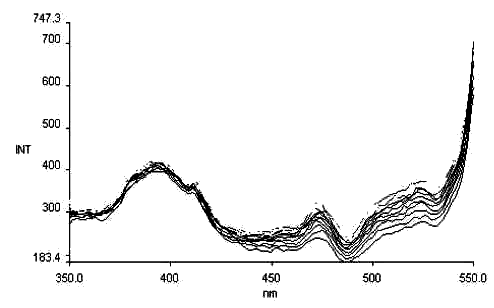
Fig.6 The photochromism excitation spectrum of salicylaniline
lem = 620nm
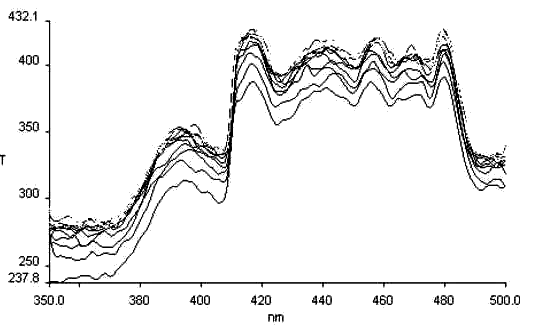
Fig. 7 The
photochromism excitation spectrum of salicyl-o-diaminobenzene
lem = 560nm
2.4 Thermochromism
The condition is that we have heated the solid state of sample to 60ºC, then quickly detected them in the fluorescence spectrometer.
Less papers on thermochromism of Schiff bases are reported, we have dynamically detected thermochromism of salicyl-p-diaminobenzene (see Fig.9), and salicyl-o-diaminobenzene (see Fig. 10). It is found there is the same basic law for thermochromism as that for photochromism, and so we think a certain relationship must be between thermochromism and the molecular structure.
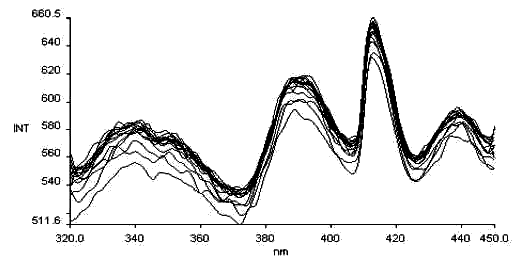
lem= 500nm
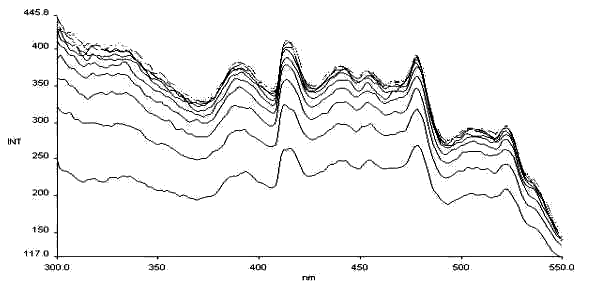
Fig. 9 The thermochromism excitation spectrum of salicyl-p-diaminobenzene
lem = 576nm
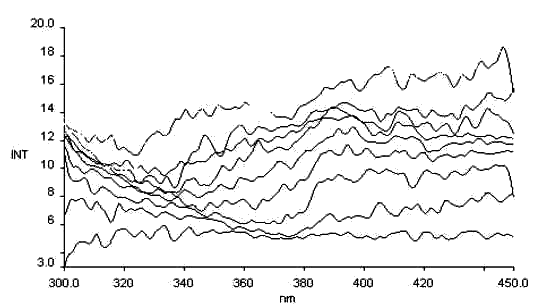
Fig.10 The thermochromism excitation spectrum salicyl-o-diaminobenzene
lem = 560nm
It is found in the test that after exposed to light or heat, the absorption spectra of the Schiff bases from salicylaldehyde and amines obviously change. No photochromism is found for the Schiff bases from benzaldehyde and amines. This seems to relate to the fact that the structure of the former is readily changed, while that of the latter is relatively stable. For organics, photochromism, or thermochromism is concerned with change of the molecular structure, e.g., tautomerizm, cis-trans isomerism, ring-opening or ring-closing reaction, on occasion, dimerization or oxidation-reduction reaction. Perhaps, there is a triangular relationship among thermochromism, photochromism, and the molecular structure.
REFERENCES
[ 1] Keitaro N, Jacques D. Chemistry of materials ( Huaxue Cailiao)
,1997, 9 (12): 2682.
[ 2] Barbara P, Rentzepis P, Brus L. Journal of America Chemical
Society (Meigu
Huaxue Huizhi), 1980, 102: 2786.
[ 3] Mitra S, Tamai N. Chemical Physics Letters ( Huaxue Wuli
Tongxun) ,1998, 282: 391.
[ 4] Zhao Jianzhang, Zhao Bing, Lia Juzheng et al. Chemical Journal of Chinese Universities (Gaodeng Xuexiao
Huaxue Xuebao), 2001, 22 (1): 136.
กก