http://www.chemistrymag.org/cji/2003/053018pe.htm |
Mar. 1, 2003 Vol.5 No.3 P.18 Copyright |
Liu Yinghai, Liu Zhanjun, Liu
Xiaohui, Deng Kuilin #
(College of Chemistry & Environmental Science,
Hebei University, Baoding, 071002; #Institute of Chemistry, Chinese Academy of
Sciences, Beijing, 100080, China)
Received on Nov.1, 2002.
Abstract Graft cellulose- polymethylacrylate copolymers have been obtained in aqueous alkaline medium. The copolymerization was carried out using a redox system-potassium diperiodatocuprate (III) [Cu (III)]- cellulose system as an initiator. The various reaction conditions were investigated at different initiator concentration and varying pH of the reaction system, the ratio of monomer to cellulose, the temperature and the length of time of copolymerization. It was found that the grafting percentage and grafting efficiency were relatively higher than those of other methods, indicating that [Cu (III)]- cellulose system is an efficient redox initiating system for cellulose graft copolymerization. The optimum conditions for affording maximum grafting have been evaluated. The structures and the thermal stability of cellulose and cellulose- g- PMA were individually characterized by infrared spectroscopy (IR) and thermogravimetric analysis (TGA). The scanning electron microscope (SEM) photographs and X- ray diffraction spectra indicate that the graft copolymer has been largely modified in the microstructure compared with the cellulose. A mechanism is proposed to explain the generation of radicals and the initiation.Keywords potassium diperiodatocuprate (III) [Cu (III)]; cellulose; methyl acrylate; graft copolymerization
1 INTRODUCTION
Cellulose is a natural macromolecule, and has been
applied widely to our daily lives, the fields of industry and agriculture. Especially, due to abundant source of cellulose
on the earth, cellulose is largely used as a raw material to reduce the costs in producing
some expensive goods. Ushakov [1] first reported cellulose graft copolymers in
1943. Since then, an ever-increasing attention has been paid to the study of cellulose
graft copolymers. Nowadays, it is no doubt that cellulose graft copolymers are cheap
modified products, which possess extensively commercial uses.
As it is well known, graft copolymerization of cellulose and other
natural macromolecules can be initiated by transition metal ions [2-5].
Especially, Ce (IV) was widely used in the graft modification of cellulose [6],
casein [7],chitosan [8] and starch [9]. But its high
price and lower initiation efficiency limit the scope of its application. In our early
works, we studied the natural macromolecule graft copolymers such as chitosan with methyl
acrylate [10], casein with butyl acrylate [11], starches with methyl
acrylate [12]. These works were proved very successful and certified that Cu
(III) is an efficient initiator in grafting methyl acrylate (MA) or methyl methacrylate
(MMA) onto macromolecules.
Though many graft copolymers of cellulose have been synthesized, the grafting of
methyl acrylate, an industrially important monomer, onto cellulose using Cu (III) as an
initiator has not been reported. In order to make a further investigation to the properties of Cu
(III) and expand the application to cellulose, this paper will present the study on the
graft copolymerization of methyl acrylate onto cellulose with Cu (III) as an initiator.
2 EXPERIMENTAL
2.1 Materials
2.2 Graft Copolymerization and Treatment of Copolymer
Graft copolymerization was carried out in a 50 mL four- necked flask equipped with thermometer, condenser, stirrer and gas inlet. In a typical reaction, 0.3g cellulose and distilled water was added with constant stirring under nitrogen. The required amount of monomer MA was added, followed by Cu (III) aqueous solution and the total volume was made up to 20 mL. The graft copolymerization was performed on the different conditions of initiator concentration, monomer concentration, different pH, temperature and the span of time. After the completion of reaction, the reactant was cooled and neutralized by aqueous hydrochloric acid solution. Then it was filtered through weighed sintered glass funnel, washed to neutral and dried to a constant weight under vacuum at 70ºC. The total weight of PMA, including the weight of homopolymer of MA and PMA grafted on Cellulose, was calculated from the weights of the product after drying and the cellulose charged before reaction. The homopolymer of methyl acrylate was removed from the crude graft copolymer by exhaustive Soxhlet extraction with acetone for 48 hours. The final copolymer was then dried to a constant weight under vacuum.
2.3 Measurements
Cellulose- g- PMA was characterized, after exhaustive Soxhlet extraction to remove PMA, by IR analysis using an FTS- 40 spectrophotometer (BIO-RAD Co. U.S.A.) in the potassium bromide pellets. The TGA of cellulose (2.75 mg) and the copolymers (2.58 mg) were carried out on a Shimadzu apparatus DGC-40 DTA-TG in nitrogen atmosphere at a heating rate of 10ºC/min. A scanning electron microscope, AMKAY- 1000B, and a Yaa 900 X- ray diffractometer was used to observe the morphologies of cellulose fibers and cellulose- g- PMA fibers.
3 RESULTS AND DISCUSSION
The grafting parameters, such as total conversion percentage (C %), grafting efficiency (E %) and grafting percentage (G %) were defined and calculated as follows:
C % = (total weight of PMA/ weight of MA charged) กม100 %
E % = (weight of PMA grafted/ total weight of PMA) กม100 %
G % = (weight of PMA grafted/ weight of cellulose) กม100 %
3.1 Effect of the concentration of Cu (III)
The influence of the concentration of Cu (III) on the grafting parameters was shown in
Fig. 1. With increasing of [Cu (III)], C% and G% increase sharply when the concentration
of Cu (III) is lower, whereas, E % changes little. This behavior means that Cu (III) is
mostly used for the generation of free radicals on the cellulose backbone for the
initiation of the graft copolymerization. More and more macroradicals could be originated,
as a result, C % and G % augment. The active sites may be increased on cellulose backbone
for grafting at higher concentration of the initiator. Cu (III) is able to participate in
the termination reactions between Cu (III) and macroradicals, which causes the decline of
C% and G%. At the same time, so many macroradicals could accelerate the homopolymerization
leading to the decrease of E%.
3.2 Effect of pH
As shown in Fig.2., the grafting parameters were evaluated under the conditions of different pH. The required amount of aqueous hydrochloric acid solution or aqueous potassium hydroxide solution was added to the reaction system before Cu (III) was injected to make pH vary, so the graft copolymerization could be carried out at different pH. The variation of pH causes the change of C %, E% and G % because of existence of different complex forms of Cu (III) at different pH. In alkali aqueous solution, the ratio of the concentration of H3IO62- to H2IO63- changes with pH [14, 15], which leads to the different activity of the Cu (III) complexes and directly influences the amount of radicals in the reaction system. The optimum pH for maximum grafting MA onto cellulose is at 11.6.
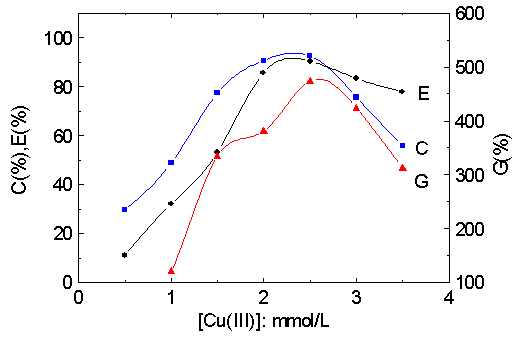
Fig. 1 Effect of [Cu (III)] on graft parameters.
MA/cellulose: 9.5; pH: 11.6; 35ºC; 1 h.
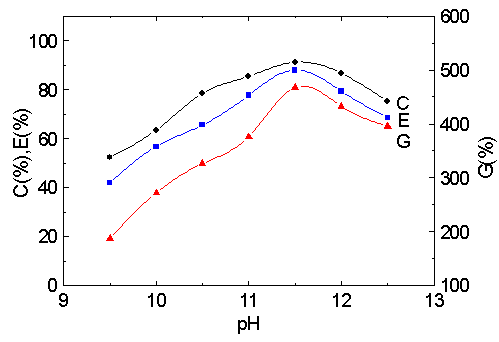
Fig. 2 Effect of pH on graft parameters.
MA/cellulose: 9.5; [Cu(III)]: 2.5กม10 -3 M ;35ºC; 1 h.
3.3 Effect of the ratio of MA to cellulose
The influence of the ratio of MA to cellulose on the graft parameters was studied and
shown in Fig. 3. It is found that G % and C % reach a maximum when the ratio of MA to
cellulose is added up to 9~10 and then fall gradually. Because of the limited solubility
of MA in the reaction medium, when the ratio of MA to cellulose is lower, the collision
probability between cellulose and MA molecule is less so that C% and G% is lower too. With
an increase of the ratio of MA to cellulose, cellulose- g- PMA could play a role as self-
emulsifier so as to adsorb more monomers on cellulose surface, which accordingly enhances
the rate of the graft reaction, therefore, C% and G% increase sharply. If the ratio of MA
to cellulose exceeds 10, the adsorption of monomers onto cellulose is so much as to
interfere largely with the approach of both Cu (III) and cellulose, which is necessary for
initiation, therefore, C% and G% decline. When the ratio of MA to cellulose is higher, the
chain transfer reactions will be accelerated, which makes E% decrease gradually.
3.4 Effect of the temperature
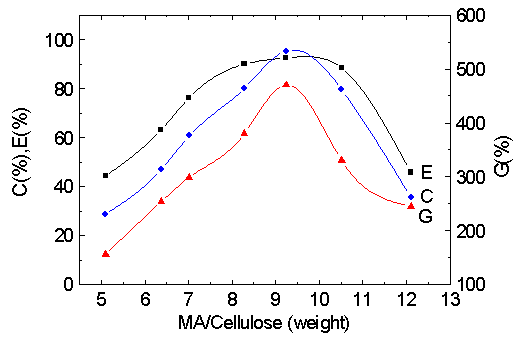
Fig. 3 Effect of MA/cellulose on graft parameters.
[Cu (III)]: 2.5กม10 -3 M; pH: 11.6; 35 กใ C; 1.5 h.
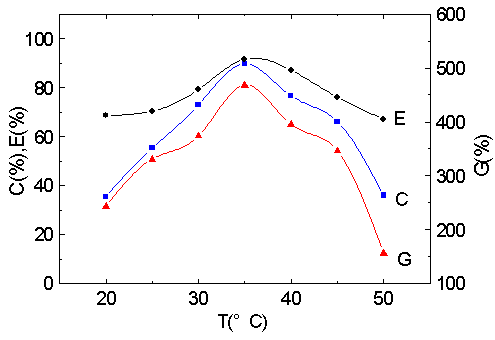
Fig. 4 Effect of
temperature on graft parameters.
MA/cellulose: 9.5; [Cu (III)]: 2.5กม10 -3 M; pH: 11.6; 1.5 h.
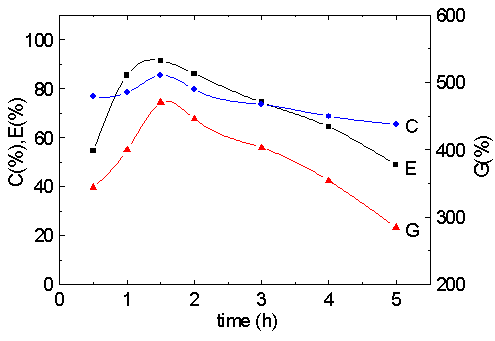
Fig. 5 Effect of reaction time on graft parameters.
MA/cellulose: 9.5; pH: 11.6; [Cu (III)]: 2.5กม10-3 M; 35
Fig. 5 illustrates the influence of the reaction time on graft parameters. As expected, both the grafting percentage and the grafting efficiency increase in value with increasing the reaction time first. However, the prolonged reaction time does not improve them after 1.5 hours. The reason can be attributed to the following fact that the reaction is carried out in alkali medium, the amount of the hydrolysis of carbonyl group increases with the prolonged time and leads to the decrease of pH. So, prolonging the reaction time obtains the similar results in contrast with the reaction temperature.
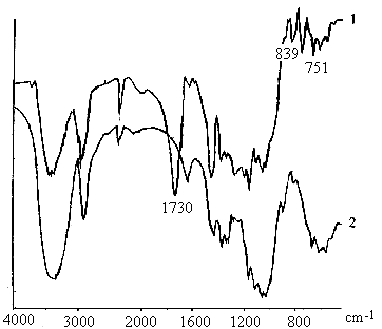
Fig. 6 Infrared spectra of cellulose-g-MA (1) and cellulose (2) 3.6 IR spectral studies
The grafting was confirmed by comparing the IR spectrum of cellulose with that of the grafted product and the results obtained are shown in Fig.6. The main difference observed is the appearance of a carbonyl absorption band at 1730 cm-1 corresponding to the carbonyl group of PMA chains. Absorption bands at 839 cm-1 and 751 cm-1 are observed due to the rocking absorption of methylene groups in PMA. All these bands are absent in the IR spectrum of pure cellulose. Owing to the results above, it could be proposed that Cu (III) may react with -OH group in cellulose to originate macroradicals first and then initiate MA grafting polymerization. Obviously, it is demonstrated that the final product is a graft copolymer of cellulose and MA.
3.7 Thermal analysis
Thermogravimetric analysis (TGA) of pure cellulose and the grafted copolymer is shown in Fig. 7. The TGA of cellulose shows a weight loss in two stages. The first stage ranges from 20ºC to 90ºC and shows about 4.09 % loss in weight. This may be assigned to the loss of adsorbed and bound water. The second stage of weight loss starts at 258.6ºC and continues up to 501.6ºC, during which there was 81.13 % of weight loss due to the degradation of cellulose. The TGA of the grafted product is different from that of cellulose. It is observed that the grafted copolymer only has one stage of distinct weight loss between 260.1ºC and 401.1 ºC with about 90.11 % of the weight loss, which is attributed to the degradation of grafted polymer. It is evident that grafting MA onto cellulose can improve the thermal stability of pure cellulose. Due to the presence of PMA, the copolymer enhances hydrophobic character compared with that of pure cellulose.
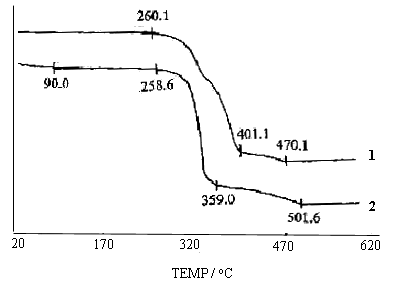
Fig. 7 Thermograms of cellulose (2) and cellulose-g-PMA (1)
3.8 Study of scanning electron microscope
Fig. 8 demonstrated the SEM micrograph change of cellulose and cellulose-g-PMA. Compared
with pure cellulose, the morphology of graft copolymer changed obviously. It can be seen
that
 (1)
(1) 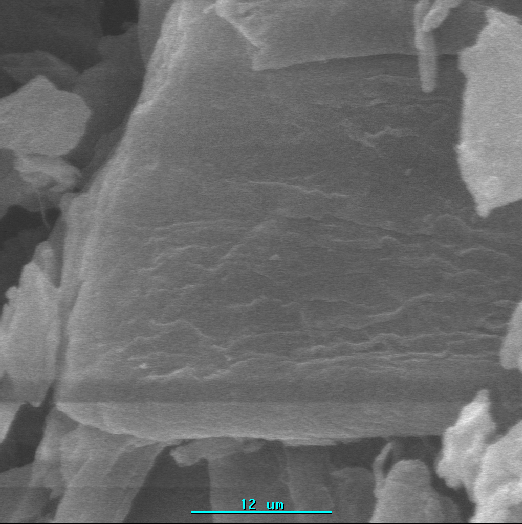 (2)
(2)Fig. 8 SEM micrographs of cellulose-g-MA (1) and cellulose (2)
3.9 Analysis X- ray diffraction
The X- ray diffraction spectra of pure cellulose and cellulose- g- PMA were measured as
shown in Figure 9. It is obvious that the X-ray diffraction spectrum of the native
cellulose has three sharp peaks, but after the cellulose was grafted with MA, it only
shows two blunt peaks. By calculation, it can be obtained that the crystallinity of
cellulose is 0.423, and that of cellulose- g- PMA is 0.132. The result manifested that the
crystal phase was also involved in the grafting reaction besides the amorphous phase.
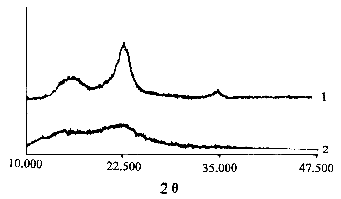
Fig. 9 The X- ray diffraction spectra of cellulose- g- PMA (2) and pure cellulose
(1).
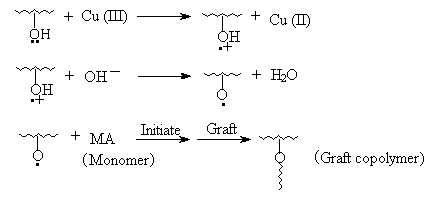
3.10 The initiation mechanism of grafting
reaction
The above results illustrated that MA has been grafted onto cellulose. So, it can be
concluded the single electron produced in the process of Cu (III) -> Cu (II) is able to
initiate cellulose to graft MA. The IR spectrum above has characterized the structure of
graft copolymer. So the initiation mechanism referring to the paper may be shown as
follows:
4 CONCLUSION
The feasibility of grafting MA onto cellulose by using Cu (III) as the redox initiator has been demonstrated by this work. The thermal stability of grafted product has been improved greatly and the microstructure changes of graft copolymers were very obvious. The conclusions are obtained as follows: First, Cu (III) obtained from CuSO4·5H2O is cheaper than other initiators; hence, it is suitable to popularize its application. Second, graft copolymers with high grafting efficiency and grafting percentage have been produced. Cu (III) is concluded to be an efficient redox initiator for the graft copolymerization of cellulose and MA. Moreover, because the activation energy of the reaction employing Cu (III) as initiator is low so that the graft copolymerization is able to be carried out at a mild temperature (35ºC) and in alkali aqueous medium, which is superior to other initiators. So, Cu (III) used as initiator is thought to be practical and has a good foreground.
ACKNOWLEDGEMENTS
The authors are indebted to the Natural Science
Foundation of Hebei Province for the financial support of this research.
REFERENCES
[1] Ushakov SN, Fiz-Mat. Nauk (USSR), 1943,
1: 35.
[2] Liu Yinghai, Song Xingru, Shi Hongmei, Xu Lili.
Chem. J. Chinese Univ., 1990, 11 (3): 328.
[3] Gao Jianping, Yu Jiugao, Wang Wei et al. J. Macromol. Sci., Pure Appl. Chem., 1998, A
35 (3): 483.
[4] Liu Yinghai, Liu Weihong, Yang Jiming et al. J. Molecular Sci., 1996, 12 (2): 190.
[5] Mazet M M, Ayele J, Rigaudie I. Water Res., 1992, 26 (4): 409.
[6] Su Maoyao, Ding Xinying. J. South China. Univ. Tech., 1994, 22 (6): 118.
[7] D Mohan, G Radhakrishnan, S Rajadurai et al. J. Appl. Sci. Polym. Chem. Edition.,
1984, 29: 329.
[8] Dinesh K Singh, Alok R Ray. J. Membrane Sci., 1999, 155: 107.
[9] George F F, Robert C B, Doane W M. J. Appl. Sci. Polym. Chem. Edition., 1983, 21:
2095.
[10] Yinghai Liu, Zhenghao Liu, Yanzhe Zhang et al. J. Macromol. Sci., Pure Appl. Chem.
2002, A39 (1&2): 129.
[11] Yinghai Liu, Yangzhe Zhang, Zhenghao Liu et al. J. Eur. Polym. 2002, 38 (8): 1619.
[12] Yinghai Liu, Jinsong Zhang,
Weiping Li. J. Hebei Univ., 2001, 21 (1): 57.
[13] Taiswal P K, Yadava K L. Indian J. Chem., 1973, (11): 837-841.
[14] Crouthamel C E, Meek H V, Martin D S et al. J. Am. Chem. Soc., 1949, 71: 3031-3035.
[15] Crouthamel C E, Meek H V, Martin D S. J. Am. Chem. Soc., 1949, 73: 82.
กก