http://www.chemistrymag.org/cji/2003/055037ne.htm |
May 1, 2003 Vol.5 No.5 P.37 Copyright |
Yang Fang, Zheng Wenjie, Bai Yan, Feng
Dexiong, Ouyang Jianming
(Department of
Chemistry, Jinan University, Guangzhou 510632, China)
Received Nov. 7, 2002; Supported by the Natural Science Foundation of Guangdong Province, China (970630)
Abstract It was discovered by our team a
novel fluorescence characteristic of unconjugated bilirubin in NaOH solution. Fluorescence
and UV-visible spectra characteristics of unconjugated bilirubin and transition metal ions
Zn2+, Cu2+ in NaOH solution were further investigated. The results
indicated that zinc ion impacted a distinct enhancement and cuprum ion quenching on the
fluorescence of bilirubin. The fluorescence quenching could be reduced under the condition
of high concentration of zinc ion, which was used to develop the fluorimetry of bilirubin
in high concentration of zinc ion, and to determine bilirubin in Chinese medicine. The
UV-visible spectrum also showed that zinc ion was prior to cuprum ion to interact with
bilirubin in Bilirubin-Zn-Cu (BR-Zn-Cu) system.
Keywords Unconjugated bilirubin, Zinc ion, Cuprum ion, UV-visible spectrum,
Fluorescence
1. INTRODUCTION
Bilirubin (BR for short) and biliverdin (BV for short) are important bile pigments. In
mammals, BR is deacidized to BV by BV reductase. BR is both the internal toxin and
antioxidant in human body and has positive effects to the regeneration of hepatic cells.
BR is also the main ingredient in cow bezoar which is a famous Chinese medicine. Together
with its complexes, BR is closely related to the formation of gallstone[1-2].
As a result, much attention has been paid to the study of the interactions between BR and
metal ions [3-11].
But many researches on BR were performed in organic solvents. As to the
fluorescence of BR, it was rarely studied because of its low fluorescence intensity in
aqueous solution and organic solvents. In 1996, J. R. Ferraro[1] studied the
coordination between Cu2+ and BR in NaOH by FT-IR and EPR.
The fluorescence of BR had been studied by our team and it was
discovered that unconjugated BR had a novel fluorescence peak in NaOH solution at room
temperature[12]. Its excitation wavelength was 464nm and emission wavelength
was 524nm, both of which would not shift with the change of the concentration of NaOH,
illumination, and heat treatment of BR. But the fluorescence intensity would change
greatly. The fluorescence characteristics of BR were quite similar to that of BV, and the
excitation wavelength of BV was 465nm, the emission wavelength being 525nm. The
interaction between various metal ions and BV led to three kinds of changes in the
fluorescence spectrum of BV: Zn2+ and Cd2+ had a distinct
fluorescence enhancement on BV; Cu2+ and Hg2+ caused fluorescence
quenching; Li+, Ba2+, Mn2+, Fe3+, Co2+,
Ni2+, Pb2+, Bi3+, La3+, Ce3+ and Zr4+
had no effects on the fluorescence of BV. Therefore Zn2+ and Cu2+
were chosen to be studied in this paper. So were the fluorescence and UV-visible spectra
of BR-M (Zn2+, Cu2+) system in NaOH solution at room temperature.
The fluorimetry of BR in high concentration of zinc ion was also developed because the
Chinese medicine cow bezoar included a quantity of metal complexes of BR which were active
constituents in cow bezoar. This method made Zn2+ as a good factor and reduced
the disturbance from other metal ions, and it could be applied to the determination of BR
in Chinese medicines.
2. EXPERIMENT
2.1 Instrumentation
Throughout the experiments a Shimadu (Kyoto Japan) UV260 spectrum recording meter was
used. The fluorescence experiments were carried out with a 970-MC fluorescence
spectrometer (The Third Analytical Instrument Factory of Shanghai, China).
2.2 Reagents
Bilirubin was obtained from Sigma Chemical Company. Zn (Ac)2 , Cu (Ac)2
and NaOH were all made in China and analytically pure. Double distilled water was used for
the preparation of all solutions. An accurate quantity of BR was dissolved into 1.2mol¡Á L-1
NaOH to form the aqueous alkaline solution of BR, which was called fresh BR solution.
2.3 Heat treatment of BR solution
Fresh BR solution was heated at a constant temperature and the temperature was controlled
at 75¡À0.1¡ãC, then it was cooled by water to room temperature.
2.4 Adding Zn2+, Cu2+
After heat treatment of fresh BR solution, Zn (Ac)2 and Cu (Ac)2
solutions were added with different ratios. Then fluorescence intensity was examined at lex=464nm and
3. RESULTS AND DISCUSSION
3.1 Fluorescence of Zn2+, Cu2+ and BR
Table 1 was fluorescence intensities of BR and different concentration of Zn2+.
The concentration of BR was 5mmol¡Á L-1 in this table. It could be figured out that
the fluorescence intensities were greatly enhanced when Zn2+ was added to NaOH
solution of BR. And the changes of fluorescence intensities were not so great when the
ratio of BR and Zn2+ was varied from 1:1 to 1:10000. So Zn2+ had a
distinct fluorescence enhancement to BR and the ratio of coordination between BR and Zn2+
was 1:1.
| Tab. 1 Fluorescence
intensities at different concentration ratio |
Tab. 2 Influences of Zn2+, Cu2+ on fluorescence intensity of BR | ||||||||||||||||||||||||||||||||||||||||||||
¡¡
|
¡¡
|
||||||||||||||||||||||||||||||||||||||||||||
Table 2 showed the fluorescence intensities of BR-Zn-Cu system. BR was in unit of mmol¡Á L-1. The ratio of three substances was the same, i.e., cZn
2+:cCu2+:cBR =10000:10:1. But the sequences of being mixed together were different. The ratio sequences listed in Table 2 were the mixing sequences. Table 2 indicated that fluorescence quenching of BR caused by Cu2+ could be reduced under the condition of high concentration of Zn2+, which was not influenced by the mixing sequences of the three substances.3.2 UV-visible spectra of Zn2+,
Cu2+ and BR
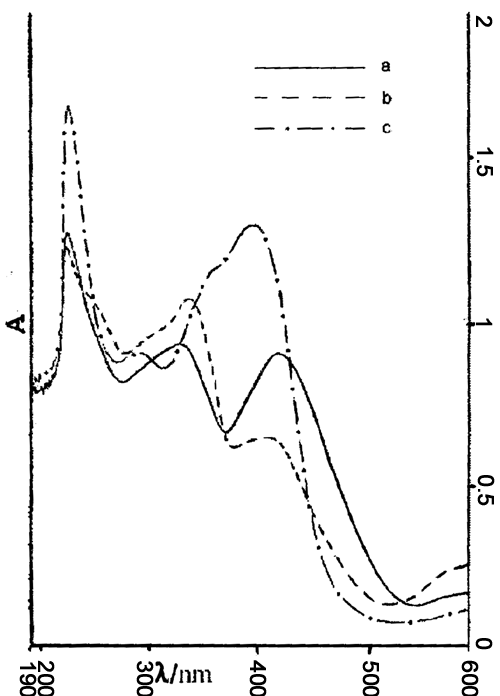
The color of fresh aqueous alkaline solution of BR was orange. It had three absorption
peaks (see Fig. 1a) respectively at 421.4nm, 329.6nm, 224.4nm. The peak 421.4nm was
related to p ¡úp* transition of two pairs of pyrrole
rings; the peak 329.6nm was contributed to n¡úp * transition; and the narrow high peak 224.4nm was due to s ¡ús * transition.
|
Fig.1 UV-visible Spectra of BR, BR-Zn and BR-Cu |
The orange color would change into dark green when Zn2+ was added to the fresh BR solution. With the increasing of concentration of Zn2+, blue shift would happen at the peak 421.4nm. Fig. 1b was cBR:cZn
2+=1:1. When 1a was compared with 1b, the peak at 421.4nm in 1a shifted to 407.2nm in 1b, and the intensity reduced greatly. This peak would disappear if the concentration of Zn2+ was increased. And yet red shift happened at the peak 329.6nm and the intensity increased gradually. This peak shifted to 335.0nm in fig. 1b. This demonstrated that BR was oxidized to BV and complex BV-Zn was formed after the interaction between Zn2+ and BR.When Cu2+ was added to the fresh BR solution, the orange color also changed into dark green. Fig. 1c was cBR:cCu2+=1:1. When 1a was compared with 1c, the peak 421.4nm in 1a shifted to 395.0nm in 1c and the intensity increased. The peak 329.6nm in 1a also shifted to 290.0nm in 1c, but the intensity reduced greatly. And it would disappear when the concentration of Cu2+ was ten times as much as that of BR. This also demonstrated that Cu2+ and BR were coordinated and consequently complex BR-Cu was formed. Because of the oxidation of Cu2+, complex BV-Cu was also formed.
The UV-visible spectra of BR, Zn2+ and Cu2+ in the same solution were further studied. The ratio and the mixing sequences in Table 3 were the same as that in Table 2.
It could be seen from the figures in Table 3 that the peak 386.2nm in Table 3a was very weak and blue-shifted obviously if compared with the peak of fresh BR at 421.4nm, which was similar to the spectrum of BR and Zn2+. The concentration of Zn2+ was 1000 times as much as that of Cu2+. Although the peak 386.2nm was weak, it did not disappear for it was related to the influence of Cu2+. So in this system Zn2+ and Cu2+ both interacted with BR, and the interaction of Zn2+ was more distinctive because the concentration of Zn2+ was higher than that of Cu2+.
The peak 340.2nm (intensity being 0.094) in 3a was quite similar to the peak 338.8nm (intensity being 0.099) in 3b. And the peak 386.2nm in 3a had disappeared in 3b. It was similar to the spectrum of BR and Zn2+. So in this system Zn2+ first interacted with BR and it was Zn2+ that produced the main effect.
The intensities of two peaks, 397.0nm and 357.4nm in 3c, were both 0.103. Because Cu2+ first coordinated with BR, the peak 421.4nm of fresh BR blue-shifted to 397.0nm and its intensity increased. However, when Zn2+ was added, Zn2+ competed with Cu2+ to interact with BR, which caused the peak 329.6nm of fresh BR to red-shift to 357.4nm and the intensity also increased.
A conclusion could be drawn from the above result that the high concentration of Zn2+ was prior to Cu2+ to interact with BR in NaOH solution.
Tab. 3 UV-visible Spectra of BR-Zn-Cu system
absorption peak (intensity) |
||||
a |
cZn 2+:cCu2+:cBR=10000:10:1 |
386.2nm (0.060) |
340.2nm (0.094) |
224.6nm (0.760) |
b |
cZn 2+:cBR:cCu2+=10000:1:10 |
------ |
338.0nm (0.099) |
224.6nm (0.764) |
c |
cCu 2+:cBR:cZn2+=10:1:10000 |
397.0nm (0.103) |
357.4nm (0.103) |
224.4nm (0.770) |
3.3 Fluorimetry of bilirubin
The fluorescence quenching of bilirubin by cuprum ion could be reduced under the condition
of high concentration of zinc ion, which was utilized to develop the fluorimetry of
bilirubin in the presence of zinc ion. After the heat treatment of fresh BR solution, Zn2+
was mixed into it. The concentration of Zn2+ in the mixed solution was
0.1mol¡Á L-1. Fluorescence intensities of different concentration of BR were
recorded and the regression equation was F=1.56+3.65c, where F was the fluorescence
intensity and c was the concentration of BR in unit of
The recovery of two Chinese medicines detoxicating tablet of cow bezoar and zhongsheng wan were determined. First, the medicines were peeled and ground to powder. Second, absolute ethyl alcohol was added to the powder. After being thoroughly mixed and slightly heated, the mixture was centrifuged and the solution was poured out which was yellow. Third, 1.2mol¡Á L-1 NaOH was added to the remaining solid substance to extract BR. Then the mixture was centrifuged for the second time and the deposited matter was gotten rid of. After the heat treatment of NaOH solution which contained BR now, Zn2+ was added to it and the concentration of Zn2+ in mixed solution was 0.1 mol¡Á L-1. Finally, the mixed solution was equally separated into two. One was determined by fluorescence directly; the other was added in an accurate quantity of BR, which was also after heat treatment, and then the fluorescence was determined, recovery being computed. Since BR could not be dissolved in absolute ethyl alcohol, the quantity of BR would not lose during the procedure. The average recovery ratio of detoxicating tablet of cow bezoar was 94.60% (RSD = 1.48%, n=8) and the average recovery ratio of zhongsheng wan was 96.65% (RSD = 1.37%, n=6).
Fluorimetry of BR was presented in the condition of high concentration of Zn2+. This method made Zn2+ as a good factor and reduced the disturbance caused by the fluorescence quenching of Cu2+. It was a convenient and accurate method to determine the concentration of BR in medicine.
4. CONCLUSIONS
The mechanism of fluorescence enhancement of BR caused by Zn2+ was
approached as follows: Intramolecular hydrogen bond and intermolecular hydrogen bond could
be easily formed due to the ligand atoms and coordination groups in BR molecule. And
intramolecular hydrogen bond often led to fluorescence quenching. In the aqueous alkaline
solution of BR, ionization happened on both the proton of carboxylic group and the proton
of nitrogen atom in pyrrole. So intramolecular hydrogen bond in BR molecule had been
broken to some degree. When Zn2+ was added to it, Zn2+ also broke
intramolecular hydrogen bond and strengthened the flatness of conjugate system. Therefore,
conjugate p bond was
lengthened in favor of p ® p * transition. On the other hand, when BR did not coordinate with
metal ions, it had more degree of freedom and was easily to deform. If the deformation
happened to molecules in excited state, energy band crossing would happen between ground
state and excited state, and so would the multi-phonon process of nonradiation transition.
The coordination both broke the intramolecular hydrogen bond and strengthened the rigidity
structure of molecule. As a result the deformation was difficult to happen, and the
nonradiation transition was greatly reduced, thus fluorescence enhancement happened.
REFERENCES
[1] Ferraro J R, Wu J-G, Soloway R D et al. Applied Spectroscopy, 1996, 50 (7) : 922.
[2] Hui J B, Liu H Z, Xu Y Z. Chemical Journal of Chinese Universities (Gaodeng Xuexiao
Huaxue Xuebao), 1998, 19 (9) : 1359.
[3] Bonnett R, Davis J E, Hurstyouse M B. Nature, 1976, 262: 326.
[4] Velapoldi R A, Menis O. Clin Chem, 1971, 17 (12): 1165.
[5] Doron K, Gil N. Israel Journal of Chemistry, 1983, 23(2): 177.
[6] Carra P Q. Nature, 1962, 105: 899.
[7] Fog J, Bugge A B. Nature, 1964, 203: 756.
[8] Van Norman J D, Yatsko E T. Bioinorg Chem, 1978, 9 (4): 349.
[9] He J P, Zhou X Y. Biospectroscopy, 1995, 1 (2): 157.
[10] Ouyang J M, Li C, Li Y Q et al. Thin Solid Films, 1999, 348(1-2): 242.
[11] Ouyang J M, Li C, Ling W H et al. Chinese Chemical Letters, 1999, 10 (6): 507.
[12] Zheng W J, Li C Q, Feng D X. Acta Pharmaceutica sinica (Yaoxue Xuebao), 1996, 31
(10): 785.