http://www.chemistrymag.org/cji/2003/058065pe.htm |
Aug. 1, 2003 Vol.5 No.8 P.65 Copyright |
(The Key Laboratory of Analytical Sciences of MOE and Department of Chemistry, Xiamen University, Xiamen, 361005, China)
Received Apr. 21, 2003; Supported financially by the National Natural Science Foundation of China (No.29875023), Educational Ministry Foundation of China and the Natural Science Foundation of Fujian Government.
Abstract A method of constant-wavelength
synchronous fluorescence spectroscopy (CWSF) (Dl=225nm) was developed to analyze the diprotonated form (H2TPPS2-)
and unprotonated form (TPPS4-) of meso-tetrakis (4-sulfonatophenyl) porphyrin
(TPPS) in aqueous solution simultaneously. The spectral overlap has been eliminated by the
proposed method. The pH dependences of synchronous fluorescence of H2TPPS2-
and TPPS4- have been analyzed by this method. The dissociation
equilibrium constants of TPPS in aqueous solution were obtained as pKa1 =4.76¡À0.02
and pKa2 =5.00¡À0.02.
Keywords Water-soluble porphyrin, Synchronous
fluorescence, Constant-wavelength interval, Dissociation equilibrium constant
1 INTRODUCTION
It is important to analyze the behavior of
water-soluble porphyrins in aqueous solution because of their signification in biological
processes. The porphyrin selected for our study is
meso-tetrakis(4-sulfonatophenyl)porphyrin (TPPS) (Fig. 1). Its molecular structure is
fairly rigid and has extensive electron delocalization in the nucleus. The porphyrin ring
system contains two pyrrole nitrogen atoms. The free form is called unprotonated TPPS
(TPPS4-), and when added two protons, it is called diprotonated TPPS (H2TPPS2-).
They have the dissociation equilibria in aqueous solution as follows: H2TPPS2-=HTPPS3-+H+ (pKa1), HTPPS3-=TPPS4-+H+ (pKa2).
[1,2] H2TPPS2- and TPPS4- are both highly
fluorescent in aqueous solution, so they are widely used in fluorescence analysis. The
Synchronous fluorescence spectroscopy is a good way to analyze multi-component system.[7-11] It has been developed into several variants, i.e., constant-wavelength (CW), constant-energy (CE) and variable-angle (VA) synchronous fluorescence spectroscopy etc. Constant-wavelength synchronous fluorescence spectroscopy (CWSF) is the simplest and easiest to be realized.[8,9] The excitation and emission monochromators are simultaneously scanned, separated by a constant wavelength interval, Dl. The resulting synchronous fluorescence spectra represent the intensity profile of a 45¡ã section cut through the excitation-emission matrix (EEM). The spectra is simplified, and the bandwidth is narrowed with high selectivity and good sensitivity.[12] In this paper, a quick and simple analytical method is developed to analyze the two forms of meso-tetrakis(4-sulfonatophenyl) porphyrin simultaneously without interference by using CWSF.
2 EXPERIMENTAL
2.1 Reagents
The stock solution of meso-tetrakis (4-sulfonatophenyl) porphyrin (TPPS) (Prophyrin
Products Inc.) was prepared in distilled-deionized water. The buffer solutions were
prepared by 0.01M potassium hydrogen phthalate (KHC8H4O4)
(A.R.). NaOH and HCl were used to adjust pH values. Other reagents were of analytical
grade. Distilled-deionized water was used throughout.
2.2 Apparatus
All spectra were obtained on a laboratory-constructed computer (IBM)-controlled
spectrofluorometer that was similar to the one previously described.[10,11] The
spectrofluorometer was equipped with a 350W Xenon lamp, and the slit bandpasses of
excitation and emission monochromators were set at 5nm. In addition to conventional
fluorescence spectra, the apparatus could provide all kinds of synchronous spectra. The pH
values were measured by using Delta320pH meter (Mettler Toledo). An optic glass cuvette
with a pathlength of 1¡Á1cm was used throughout. The ionic strength of the solution is
controlled at 0.1M with NaCl.
2.3 Procedure
To a 10-ml volumetric flask was added a volume of 100ml TPPS with concentration of 1¡Á10-4M and 1ml NaCl with concentration of 1M. Then the solution was diluted to the mark with buffer solution of different pH value (2.40-7.50). The final concentration of 1¡Á10-6M for TPPS was used throughout. The suitable scan parameters for CWSF were selected according to the excitation and emission spectra of the two forms of TPPS. The fluorescence synchronous spectra of 1¡Á10-6M TPPS with pH from 2.40 to 7.50 were obtained with Dl=225nm. Scan rates of the excitation and emission monochromaters are set at 240nm/min.
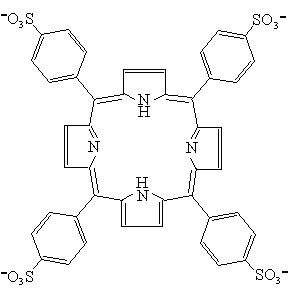
Fig. 1 The molecular structure of TPPS. Protonation occurs on the two nitrogen atoms in the porphyrin ring. 3 RESULTS AND DISCUSSION
3.1 Fluorescence spectra
The experiments showed that when pH<4, the main form in solution was H2TPPS2-, and when pH>6, the main form in solution was TPPS4-, so we used pH=2.40 and pH=6.30 to study the basic information of TPPS. The excitation and emission spectra of H2TPPS2- at pH=2.40 and TPPS4- at pH=6.30 were obtained (Fig. 2). The maximal excitation and emission wavelengths of H2TPPS2- were at 435nm and 670nm, whereas the maximal excitation and emission wavelengths of TPPS4- were at 412nm and 642nm. The results showed that the fluorescence intensity of H2TPPS2- was about three times higher than that of TPPS4-. As showed in Fig. 2, the spectra of the two forms of TPPS overlapped seriously, especially the emission spectra.

Fig. 2 Fluorescence excitation (a) and emission (b) spectra of H2TPPS2- (solid line) at pH=2.40 and TPPS4- (dotted line) at pH=6.30. The concentration of TPPS was 1.0¡Á10-6M.

Fig. 3 The CWSF spectra of H2TPPS2- at pH=2.40 (solid line) and TPPS4- at pH=6.30 (dotted line) with Dl=225nm. 3.2 Selection of the parameters of synchronous fluorescence spectroscopy
In CWSF, the most important scanning parameter is Dl. Theoretically when Dl of synchronous fluorescence spectroscopy is the Stokes shift of a substance, the intensity will be the highest and bandwidth will be the narrowest, but in fact the selection of Dl is still a result of tests considering the interference from background signal or other coexisting components.[12] A series of Dl values (223nm, 225nm, 228nm, 230nm, 233nm, 235nm) were tested.
The peak wavelengths of H2TPPS2- and TPPS4- red-shifted about 3nm, and their mutual interference of H2TPPS2- and TPPS4- became lower when Dl changed from 235nm to 225nm. It seemed that results would be good with the decrease of Dl, but when Dl was decreased to 223nm, the interference became higher again. Since the mutual interference changed not too much from 230nm to 225nm, any value could be selected in this range. In this experiment the Dl value of 225nm was used. In comparison with Fig. 2, well-resolved synchronous fluorescence spectra (shown in Fig. 3) for H2TPPS2- and TPPS4- were obtained with Dl=225nm. The synchronous fluorescence peak wavelength of TPPS4- was at 413nm and that of H2TPPS2- at 437nm. The interference between each other was lower than 5%, and the spectral distinction of Dl=225nm was the best comparing with that of other Dl values.

Fig. 4 The CWSF spectra of H2TPPS2- (A) and TPPS4- (B) changed with different pH (2.40¡ª7.50). The concentration of TPPS was 1.0¡Á10-6M.


Fig. 5 The pH dependence of the synchronous fluorescence intensity of H2TPPS2- (A) and TPPS4- (B). 3.3 Fluorescence behavior of H2TPPS2-and TPPS4- in aqueous solution with different pH
TPPS in aqueous solution in the pH range from 2.40 to 7.50 were analyzed by using CWSF (Dl=225nm), and a series of synchronous fluorescence spectra were obtained (Fig. 4). With the increase of pH, the spectral peak at 437nm decreased and the peak at 413nm increased gradually, indicating the alternation of H2TPPS2- and TPPS4- clearly. When pH was lower than 4.00, the synchronous fluorescence of TPPS4- did not appear. There was an equal fluorescence intensity point at 423nm. When pH reached 6.00, the synchronous fluorescence of H2TPPS2- completely disappeared.
3.4 Calculation of dissociation equilibrium constant
The synchronous fluorescence intensities of H2TPPS2- and TPPS4- versus pH showed Sigmoidal shapes, which were due to the dissociation equilibrium in aqueous solution. Spectrofluorimetric pH-titration curves showed that, the synchronous fluorescence intensity of the TPPS4- was not pH dependent after 6.00, and the intensity of H2TPPS2- was not pH dependent before 4.00. By using Origin6.0 professional software to fit Sigmoidal, the values of dissociation equilibrium constant were obtained as pKa1=4.76¡À0.02 (Fig. 5-A) and pKa2=5.00¡À0.02 (Fig. 5-B). This result coincided with the other spectrophotometral data listed in Table 1. Table 1. Comparison of the pKa values
Reference |
Method used |
pKa1 |
pKa2 |
1 |
spectrophotometry |
4.76¡À0.02 |
4.99¡À0.01 |
5 |
spectrophotometry |
4.86 |
4.95 |
6 |
spectrophotometry |
4.60¡À0.01 |
5.17¡À0.01 |
13 |
Not mentioned |
4.80 |
¡¡ |
This work |
CWSF |
4.76¡À0.02 |
5.00¡À0.02 |
4 CONCLUSION
In this paper, a sensitive and simple method to analyze the two forms of TPPS in aqueous
solution by constant-wavelength synchronous fluorescence spectroscopy has been developed.
It can eliminate the interference between different porphyrin forms. The technique could
provide a useful tool to study the properties of TPPS in aqueous solution. Further
application of the proposed method is under investigation.
REFERENCES
[1] Tabata M, Tanaka M. J.Chem.Soc. Chem. Commun., 1985, 1: 42-43.
[2] Qiao Q D, Gao X X. Acta Chimica Sinica (Huaxue Xuebao), 1994, 52: 595-602.
[3] Purrello R, Gurrieri S, Lauceri R. Coord Chem. Rev., 1999, 190: 683-706.
[4] Papkovskii D B, Savitskii A P, Ponomarev G V. Appl.Spectrosc., 1989, 51: 786-790.
[5] Itoh J, Yotsuyanagi T, Aomura K. Anal. Chim. Acta., 1975, 74: 53-60.
[6] Okumura R, Hinoue T, Watarai H. Anal.Sci., 1996, 12: 393-397.
[7] Murillo Pulgarín J A, Alaón Molina A, Fernández López P.
Anal. Chim. Acta., 1998, 370: 9-18.
[8] Garcia L, Blazquez S, San Andres M P et al. Anal. Chim. Acta, 2001, 434 (2): 193-199.
[9] Wang L Y, Zhou Y Y, Wang L et al. Anal.Chim. Acta, 2002, 466 (1): 87-92.
[10] Li Y Q, Huang X Z. Fresenius J. Anal. Chem., 1997, 357: 1072-1075.
[11] Li Y Q, Sui W, Wu C et al. Anal. Sci., 2001, 17: 167-170.
[12] Cheng G Z, Huang X Z, Xu J G et al. Fluorescence Analysis (Yingguang Fenxifa). 2nd
Ed., Beijing: Science Press, 1990: 201.
[13] Zeng Y E, Zhang H S, Chen Z H. Handbook of Modern Chemical Reagent (Xiandai Huaxue
Shiji Shouce), Inorganic Ion Color Reagent (Wuji Lizi Xianseji). Beijing: Chemistry
Industry Press, 1989: 785.
¡¡
¡¡