http://www.chemistrymag.org/cji/2003/058066ne.htm |
Aug. 1, 2003 Vol.5 No.8 P.66 Copyright |
Liu
Xiuying, Ma Zhiguang, Shen Shigang, Shan Jinhuan, Du Baoan
(College of Chemistry & Environmental Science, Hebei University, Baoding 071002 China)
Received May 29, 2003.
Abstract Phase transfer catalyst
PEG-400 was used in the synthesis of N-benzylphthalimide under microwave irradiation. Several
influence factors on the product yield were investigated, such as the proportion of
reagents, the irradiation time, the quantity of DMF and PEG-400 used. The experimental
conditions were optimized which resulted in a high yield 98.5% within the reaction time of
120s.
Keywords N-benzylphthalimide, Microwave irradiation, Synthesis, Polyethylene
glycol-400
1 INTRODUCTION
The compounds of N-alkylating phthalimides are important intermediates in synthesizing the
fatty primary amine and a -amino acid. In the classical method, the N-alkylating
phthalimides was synthesized in N,N-dimethylformamide (DMF) by the reaction of halogenated
compound with the salt, which was prepared by the reaction of phthalimide and KOH.
Afterwards, some methods were reported such as: The phase transfer catalytic effects of
polyethylene glycols in N-alkylation[1]; convenient synthesis of N-substituted
phthalimide[2]; the reaction of N-alkylation of phthalimide by the inorganic
supported reagent[3,4]. However, reaction time of the above reported methods is
longer. Take the synthesis of N-benzylphthalimide as an example, generally the reaction
time is about 10h, the shortest is 4h and the highest yield is 94%.
N-benzylphthalimide was firstly synthesized catalyzed by the alkaline
reagent K2CO3-Al2O3 under microwave
irradiation in 2002. In this reaction, a higher yield of 98% was obtained within a shorter
time of 420s[5]. However, it took about 6-7h to prepare the catalyst (alkaline
reagents K2CO3-Al2O3) and 3h to purify the
product after the completion of the reaction. Although the reaction time is only 420s, the
work up is far from simplicity. In this respect, the preparatory method was further
improved. Synthesis of N-benzylphthalimide with polyethylene glycol-400 as phase transfer
catalyst under microwave irradiation was investigated in detail and the better result was
obtained.
2 EXPERIMENTAL
2.1 Reagents and instruments
Benzyl chloride, N,N-dimethylformamide, potassium carbonate anhydrous are all of
analytical grades. phthalimide and polyethylene glycol-400 (PEG-400) are chemical grades.
All the reagents are used without further treatment.
Microwave oven (WD750, 750W, 2450Mhz) is from Galanz Electric Limited
Company of Shunde, and Infrared spectrophotometer (FTS-40) is from BIO-RAD of America.
Melting point is measured by capillary method.
2.2 Reaction procedure
N-benzylphthalimide was synthesized from phthalimide and benzyl chloride. The reaction was
carried out with PEG-400 as phase transfer catalyst and anhydrous K2CO3 as
ordinary catalyst in DMF under microwave irradiation. The whole process could be expressed
by the following equation:
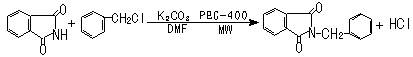
2.3 Preparation of N-benzylphthalimide
In a 50ml beaker, 1.90g phthalimide (12.5mmol), 2.0g
benzyl chloride (15.8mmol), 2.0g anhydrous K2CO3 (13.9mmol), 1.5g
PEG-400 and 2.0g DMF were mixed well and then put into the microwave oven. After
irradiated continuously for 120s, the mixture was taken out and about 30ml de-ionzed water
was added. Immediately, white crystal was formed. Then 5ml 10% aqueous NaOH was added and
stirring was continued for 5min to dissolve the rest of phthalimide. The mixture was
filtered under the reduced pressure and was washed with de-ionzed water until its pH value
was neutral. The white crystal was dried in oven (105
3.1 Influence of the proportion of reagents to yield
The relationship between the proportion of reagents and yield was shown in Table 1. When phthalimide:benzyl chloride =1:1 (molar ratio), the yield was low; when phthalimide:benzyl chloride =1:1.25 (molar ratio), the maximum yield (98.5%) were obtained (entry 4). But when the quantity of benzyl chloride was increased again, the yield was not only decreased, but also some troubles, such as filtering was not easy, excessive benzyl chloride should be treated, were led in the preparation process,
Table 1 Relationship between the proportion of reaction reagents and the yield
No. |
phthalimide: benzyl chloride (molar ratio) |
average yield (%) |
1 |
1: 1.00 |
82.7 |
2 |
1: 1.13 |
85.1 |
3 |
1: 1.19 |
89.8 |
4 |
1: 1.25 |
98.5 |
5 |
1: 1.50 |
98.5 |
6 |
1: 2.00 |
98.5 |
3.2 Influence of the irradiation time to the
product yield
The influence of irradiating time, power and temperature on the yield was investigated in
this experiment. The results showed when the microwave oven was performed at 900W the
reagents were easily coked. When the microwave oven was performed at 750W (the temperature
reached 150
Table 2. Relationship between the irradiate time and the yield
No. |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
time of irradiation (s) |
60 |
90 |
100 |
110 |
120 |
150 |
300 |
average yield (%) |
68.7 |
83.2 |
91.7 |
96.1 |
98.5 |
95.2 |
91.2 |
* 1.90g phthalimide, 2.0g benzyl chloride, 2.0g K2CO3, 2.0g DMF, 1.5g PEG-400, were added and irradiated continuously under 750W microwave oven.
3.3 Influence of the catalyst and the solvent
to the yield
As shown in Table 3 (entries 1-4), K2CO3 played an important role in
the reaction. The products were not obtained in the absence of K2CO3 .The
yield was low when 1.5g K2CO3 was added. The
good yield was obtained when 2.0g K2CO3 was added (entry
3). The yield was not increased with further increasing quantity of K2CO3.
Table 3. Influence of the catalyst and the solvent to the yield
No. |
K2CO3(g) | PEG-400(g) |
DMF(g) |
time of irradiation (s) |
average yield (%) |
Color of crystal |
1 |
0 |
1.5 |
2.0 |
120 |
0 |
|
2 |
1.5 |
1.5 |
2.0 |
120 |
76.2 |
white |
3 |
2.0 |
1.5 |
2.0 |
120 |
98.5 |
white |
4 |
2.5 |
1.5 |
2.0 |
120 |
98.5 |
white |
5 |
2.0 |
0 |
0 |
120 |
0 |
|
6 |
2.0 |
1.8 |
2.0 |
120 |
98.5 |
white |
7 |
2.0 |
1.0 |
2.0 |
120 |
83.2 |
white |
8 |
2.0 |
1.5 |
0 |
120 |
76.6 |
white |
9 |
2.0 |
1.5 |
0 |
180 |
77.9 |
white |
10 |
2.0 |
1.5 |
0 |
240 |
78.3 |
white |
11 |
2.0 |
1.5 |
0 |
270 |
Coke | ¡¡ |
12 |
2.0 |
2.0 |
0 |
120 |
70.0 |
white |
13 |
2.0 |
3.0 |
0 |
100 |
68.0 |
Light brown |
14 |
2.0 |
0 |
3.0 |
120 |
55.6 |
Light yellow |
15 |
2.0 |
0 |
3.0 |
240 |
82.6 |
Light yellow |
16 |
2.0 |
0 |
3.0 |
360 |
91.9 |
Light yellow |
17 |
2.0 |
0 |
3.0 |
420 |
98.0 |
Light yellow |
18 |
2.0 |
0 |
3.0 |
480 |
94.0 |
Light yellow |
The product was synthesized with good yield just in the presence of DMF without the participation of PEG-400. Because the DMF could offer a much greater contact area to the reacting reagents, so DMF added tended to increase the rate of the synthesis reaction. The yield of 98% could be obtained if the reagents were irradiated continuously for 420s in the presence of 2.0g DMF in microwave oven (which was not reported in the literature so far as we know). But the product was light yellow and needed to be purified (entries 14-18), and the reaction time was extended. So it is with the double actions combining of DMF and PEG-400 that the excellent results can be achieved. That is when 2.0g DMF and1.5g PEG-400 were added (entry 3).
4 CONCLUSION
In the paper, N-benzylphthalimide was successfully synthesized under microwave irradiation
and the optimum conditions were found (phthalimide: benzyl chloride:K2CO3=1:1.25:1.11,
with 1.5g PEG-400 as phase transfer catalyst and 2.0g DMF as solvent under continuous
irradiation for 120s in 750W microwave oven). The yield attained could be as high as
98.5%, and the reaction time needed was only 120s, which was shorter than the shortest
time (420s) reported. All operation cycle took only about 20min. The method was very easy
to operate, not only energy used was greatly saved, but also the reagents were reduced,
which brought the highest economic value. In addition, pollution could be further reduced.
REFERENCES
[1] Zhong Q, Shao J G, Wang J H et al. Chem. J. of Chinese Universities (Gaodengxuexiao
Huaxue Xuebao), 1987, 8 (5): 441-443.
[2] Yu S X, Zhang S, Lin X. Chem. Reagents (Huaxue Shiji), 1995, 17 (5): 310-315.
[3] Sun X H, Jin X G. Journal of Huaibei Coal Mining Teachers College (Huaibei Meishiyuan
Xuebao), 1997,18 (1): 49-53.
[4] Lu W X, Yan C G. Chem. Reagents (Huaxue Shiji), 1997, 19 (2): 125-126.
[5] Liu X Y, Qie L J, Ma Z G et al. Chemical Journal on Internet (Guoji Wangshang Huaxue
Xuebao), 2002, 4 (9): P.34
[6] Sadtler Research Laboratories. Sadtler Standard Infrared Grating Spectra. US. Sadtler
Research Laboratories INC, 1966-1977.
¡¡