http://www.chemistrymag.org/cji/2003/058067ne.htm |
Aug. 1, 2003 Vol.5 No.8 P.67 Copyright |
(Department of chemistry, Shanghai Normal University, Shanghai, 200234, China)
Received June15, 2003; Supported by the Natural science of Shanghai (No. 2000D07)
Abstract This communication reports the synthesis, crystal structure and the herbicidal activity of 1-Benzoyl-3-(4,6-disubstitute-pyrimidine-2-yl)-thiourea. The structures of the five target compounds were determined by IR, 1H NMR and MS.Keywords crystal structure, herbicidal activity, 1-benzoyl-3-(4,6-disubstitute- pyrimidine-2-yl) -thiourea
Pesticides form a major proportion of the
total agrochemicals production and usage. Approximately 4.5×109 pounds of
pesticides are used in a typical year only in USA[1]. Many acyl thiourea
compounds have been widely studied for their insecticidal, antibacterial and pesticidal
activities and promoting effect on plant growth[2-4]. These have attracted the
attention of many investigators. A series of new
1-benzoyl-3-(4,6-disubstitute-pyrimidin-2-yl)-thioureas derivatives have been synthesized
in this paper. Further, the crystal structure of Bdpt
[1-Benzoyl-3-(4,6-dimethyl-pyrimidin-2-yl)-thiourea] were determined by X-ray and its
herbicidal behavior were investigated.
The synthesis route of these compounds is summarized by the equations
below:
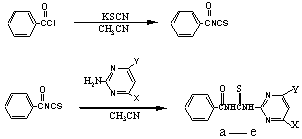
| Target compound | a |
b |
c |
d |
e |
X |
CH3 |
CH3 |
CH3 |
Cl |
Cl |
Y |
CH3 |
OCH3 |
OC2H5 |
OCH3 |
NC2H5 |
1. EXPERIMENTAL SECTION
1.1 Apparatus
The structures of the synthesized compounds were characterized by IR, 1HNMR,
MS. The IR spectra were recorded on a Jasco FT-IR spectrophotometer in the
region 4000-400cm-1. The 1H NMR spectra were obtained on a Jeol
FX-90Q spectrometer. All solids were dissolved in DMSO-d6 and TMS was taken as
internal reference. MS spectra were performed on an HP-5988-A chromatograph-mass
spectrometer. The melting points were determined on an XT4A melting point apparatus.
1.2 Reagents
2-amino-4,6-disubstitute-pyrimidine was prepared by the
literature method[5]. All other chemicals and solvents used were of AR grade.
1.3 Experimental Procedure
Preparation of the Benzoyl isothiocyanate : A mixture
of benzoyl chloride(2.81g,0.02mol), KSCN(1.96g,0.02mol) and 20ml of acetonitrile was
refluxed with stirring for 1 hr, then filtered and the filtrate was not purified for the
further reaction .During the course of reaction, the progress of reaction was controlled
by TLC.
Preparation of the 1-Benzoyl-3-(4,6-dimethyl- pyrimidine-2-yl)-Thiourea
Preparation of the single crystal of the target molecule : Its single crystal, suitable for X-ray analysis, grows from the mixed solvent of methanol and acetonitrile(1:2)by slow evaporation for 4 day.
1.4 Characterization Data for the Products
Compound (a): Yield: 85%; m.p: 196-197oC; IR(cm-1):2900,3100(N-H),1710(C=O),1240(C=S),1600(C=N).1HNMR(d:ppm):2.40(s,6H,2CH3),6.72(s,1H,Py-5-H),7.42(m,5H,Ph-H). MS (m/z): 51(83), 77(97), 105(100), 149(89), 181(42), M+286(75).
Compound (b): Yield: 75%; m.p: 196-198oC; IR (cm-1): 2950,3150(N-H), 1720(C=O), 1240(C=S), 1600(C=N). 1HNMR(d:ppm):2.40(s,3H,CH3),3.92(s,3H,OCH3),6.28(s,1H,Py-5-H), 7.25(m,5H, Ph-H). MS (m/z): 51(74), 77(99), 105(100), 165(18), 197(5), M+302(7).
Compound (c): Yield: 87%; m.p: 182-183oC; IR (cm-1): 3000,3350(N-H), 1710(C=O), 1250(C=S), 1600(C=N).1HNMR(d:ppm):1.33(t,3H,OCH2CH3),2.52(s,3H,CH3),4.29(q,2H,OCH2CH3), 6.26 (s,1H,Py-5-H),7.49(m,5H, Ph-H). MS (m/z): 51(82), 77(100), 105(98), 181(26), 213(4), M+318(14).
Compound (d): Yield: 65%; m.p: 197-198oC; IR (cm-1): 3000,3150(N-H), 1720(C=O), 1230(C=S), 1600,1580(C=N). 1HNMR(d:ppm):4.10(s,3H,OCH3),6.64(s,1H,Py-5-H),7.50(m,5H, Ph-H). MS (m/z): 51(83), 77(99), 105(100), 185(11), 217(2), M+322(8), [M+2]+324(3)
Compound (e): Yield: 54%; m.p: 164-165oC; IR (cm-1): 2950,3150(N-H), 1710(C=O), 1240(C=S),1590(C=N).1HNMR(d:ppm):1.40(t,6H,N(CH2CH3)2),3.60(t,4H,N(CH2CH3)2),6.08(s,1H,Py-5-H),7.50(m,5H, Ph-H). MS (m/z): 51(81), 77(99), 105(100), 226(6), 258(2), M+363(4), [M+2]+365(1)
2. X-RAY CRYSTAL STRUCTURE
DETERMINATION
A pale-yellow crystal of Compound (a) with dimensions
of 0.507×0.321×0.220mm was mounted in a glass capillary and data were collected at 293K
with a Bruker SMART platform ccd diffractometer crystal .Four different sets of frames
were collected using 0.30 steps in w range from 2.15 to 28.26.The exposure time was 35 per frames and
the detector to crystal distance was about 4.5 cm .The resolution of the data set was 0.84Å.
The structure was solved with direct methods .All the non –hydrogen atoms were refined anisotropically. The hydrogen atoms
were placed in idealized positions and refined by using a riding method.
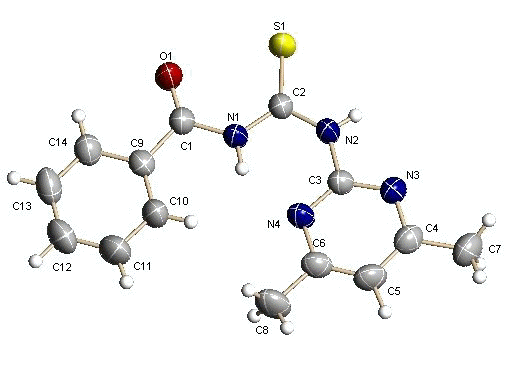
Figure 1 The crystal structure of 1-Benzoyl-3-(4,6-dimethyl-pyrimidine-2-yl)-thiourea

Figure 2 The packing crystal cell of target molecula
Data Figure2 demonstrates that the molecular structures form dimer compounds through intermolecular hydrogen bond .The bond carbonyl group C(2)=S form intramolecular hydrogen bond with N(2), whereas atom N(1) interacts with N(4) of the pyrimidine ring by intramolecular hydrogen . It should be stressed that these hydrogen bonds might possibly suggest the relation with herbicide activities by binding the site which block the transfer of electrons from the electron donor to the electron carriers.
3. HERBICIDAL EFFICIENCY
The preliminary biological tests showed that the Bdpt has excellent inhibitory activities against broad leaf (such as Amaranthus retroflexus L), meanwhile, it has part selectivity on monoctyledon plant(such as Echinochloa Crusgauis(L)).
Table 1. The Inhibition Percentage of Bdpt to kinds of weeds
Concentration |
species |
|||
Digitaria sanguinalis(L)Scop |
Chenopodium Serotinum l |
Chenopodium Serotinum l |
Amaranthus retroflexus L |
|
50 |
24.6 |
83.8 |
7.8 |
100 |
75 |
70.8 |
86.5 |
9.1 |
100 |
120 |
86.2 |
100 |
10.0 |
100 |
Acknowledgements
The X-Ray measurements were undertaken by Shanghai Institute of Organic Chemistry,
Chinese Academy of Sciences. The herbicidal efficacy was carried out by Institute of Plant
Protection ,Shanghai Academy of Agricultural Science.
REFERENCES
[1] Laspelin
[2] Noguchi T, Ohkuma K, Kosaka S. Proceeding of the 2nd International IUPAC Congress of Pesticide Chemistry, New York, 1972, 5, 263.
[3] Ramadas K, Svinivasan N. Synth. Commun, 1995, 25 (2D): 338.
[4] Lieb F, Philip U C. Chem. Plant Prot, 1994, 10: 189 (C.A.1995,122:153983k)
[5] Jin Shan Zhou, Si Jia Xue. J.Central China Norm.Univ.(Nat.Sci), 1999, 33(1): 233-237.
1-苯甲酰基-3-(4,6-二取代嘧啶-2-基)硫脲衍生物的合成、晶体结构和除草活性
薛思佳 段李平* 柯少勇 贾良斌
(上海师范大学 生命与环境科学学院 化学系 上海 200234 )
摘 要 本文报道了1-苯甲酰基-3-(4,6-二取代嘧啶-2-基)硫脲衍生物的合成、晶体结构和除草活性。标题化合物的结构经IR, 1H NMR和MS得到确证。其中对化合物1-苯甲酰基-3-(4,6-二甲基嘧啶-2-基)硫脲进行了单晶衍射,使其结构得到进一步确证。
关键词 1-苯甲酰基-3-(4,6-二取代嘧啶-2-基)硫脲,合成,晶体结构,除草活性