http://www.chemistrymag.org/cji/2003/059073ne.htm |
Sep. 1, 2003 Vol.5 No.9 P.73 Copyright |
Synthesis and spectra of two zinc salts of phosphatidic acid
Wang Jian, Liu Ping
(Fujian Institute of Research on the structure of Matter, Chinese Academy
of Science, Fuzhou 350002, China)
Received May 28, 2003; Support from the Nation Natural Science Foundation of China and
the Natural Science Foundation of Fujian province.
Abstract Two compounds
1,2-O-Di-octadecyl-sn-glycero-phophatidic acid zinc salt hydrate and
1-O-octadecyl-2-O-methyl-sn-glycero-phosphatidic acid zinc salts hydrate have been
synthesized for first time. Their IR, NMR and Mass spectra have been studied.
Keywords phosphatidic acid zinc salts, synthesis, spectrum.
1 INTRODUCTION
In 1998 year, professor Dr. Ferid Murad, Robert Fur chgott and Lewis Ignarro got the nobel
prize in physiology and medicine because they have discovered biological function of
nitric oxide as a new second messenger[1]. By their theory, Viagra drug was
putted in production, a big economic effect was got. The new age of signal drug has
already come. Besides NO, C-GMP, C-AMP etc as second messenger, the signal effects of
lipid have been noted extensively. Phosphatidic acid is one of lipid, although it is only
a little in membrane, but it plays important role. Of course, the role of phosphatidic
acid in functional activation has not been completely defined.
Intracellar stored calcium is mobilized by lysophosphatidic acid with a
G-protein-linked receptor. It activates intracellular phosphalipase C. It is an
extracellular messenger in cells. Some different cells are able to respond to
lysophosphatidic acid[2,3]. Phosphatidic acid is also a patent calcium
mobilizing stimuli. Phosphatidic acid shows active to neutrophilic leukocytes by activated
phospholipase D(PLD)[4]. Phosphatidic acid and diacyglycerol in mediating the
activation of neutrophilic leukocytes exposed to metabolic stimuli. Farther, phosphatidic
acid can increase G-protein Rac2-GDI complex dissociation, which shows as second messenger[5,6].
The effects of phosphatidic acid are in many places. Phosphatidic acid can activate some
enzymes, for example, PKC, Ca2+-dependent and Ca2+-in dependent
kinase, serine/threonine protein kinase, etc[7,8,9]. Phosphatidic acid involves
interaction between extracellular signalling pathways for example, PLC, PLA2,
IP3, etc[10,11]. The sensitivity of PI3-Kinase to PA and lyso-PA
could imply cross-talk between the phospholipase D and PI3-Kinase signal transduction
pathways in vivo[12].
To sum up, we know that phosphatidic acid is a very important lipid
compound. But phosphatidic acids are quite unstable compounds and its use was limited.
Salt of phosphatidic acid is more stable than phosphatidic acid, but there are few
synthesized sodium and calcium salt of phosphatidic acid.
The transition-metal salts of phosphatidic acid still has not been reports.
For zinc, following iron, is the second-most abundant transition metal in biology. Zinc
enzyme plays an important role in the biological catalyzed reaction and in the control of
gene expression. Besides iron salts of phosphatidic acid[13], we have
synthesized two zinc salts of phosphatidic acid and determined their molecular structure
by spectra. Their properties will be studied in the future.
2 EXPERIMENTAL
Two sodium salts 1,2-O-Di-octadecyl-sn-glycero-phophatidic acid and
1-O-octadecyl-2-O-methyl-sn-glycero-phosphatidic acid were prepared by known method(8).
Zinc chloride hydrate(ZnCl2·nH2O) was bought from Aldrich
company. The reaction equation was shown in Fig.1.
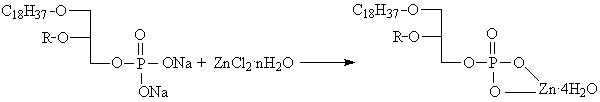
R=C18H37 (compound 1) or CH3(compound
2).
2.1 Synthesis
All reactions were performed under an atmosphere of nitrogen.
To a solution of 1,2-O-Di-octadecyl-sn-glycero-phosphetidic acid sodium
salt 0.416mmol in CHCl3(9ml) and MeOH(1ml) was added ZnCl2·nH2O
0.416mmol and the reaction mixture was stirred at 50¡æ for 20 hours, the precipitate was filtrated off and the solvent was
put into refrigerator. Light white solid 145mg was obtained. Yield of compound 1
was 42.98%. Through the same method, we got compound 2 94.2mg, the yield is 39.52%.
2.2 Element analysis The element analysis were recorded on a vario ElIII.
2.3 IR spectrum Infrared spectra of the compounds 1,2 were recorded
on a Nicolet Magna 750 Fourier transform infrared spectroscopy, respectively. The
measurement was made as KBr pellets in the region 4000~400cm-1 and scanned at
room temperature for 32 times. And in the region 600~100cm-1 we recorded
spectra as CsI pellets for 73 times scanning.
2.4 NMR spectrum 1H-NMR spectra were measured on the Varian Unity-500
superconductivity NMR spectrometer, using TMS as an internal standard, CDCl3 as
a solvent.
2.5 Mass spectrum The molecule-ion mass spectra were measured on a Finnigan MAT-312
GC/MS/DS spectrometer with electron impact(EI) method, at a resolution of 1000. The
temperature of the ion source is 160C, the gasification temperature is 250-300C, the accelerating voltage of the ion source is 3KV, the electron
energy is 70eV. Ion peaks of a series of mass spectra were collected with the computer.
3 RESULT AND DISCUSSION
3.1 Element analysis
Table 1 Elemental Analysis of Compounds 1 and 2
Compounds |
Elements(%) |
|||||
C |
H |
P |
Zn |
|||
1 |
C39H87O10PZn |
Calc. |
57.71 |
10.73 |
3.82 |
8.01 |
Anal. |
58.18 |
10.81 |
3.42 |
7.82 |
||
2 |
C22H53O10PZn |
Calc. |
46.07 |
9.25 |
5.41 |
11.34 |
Anal. |
46.77 |
8.83 |
5.19 |
11.50 |
||
3.2 IR spectrum
For compound 1 as an example, the strong absorption at 1063cm-1 could be
attributed to the stretching vibration of P-O, and the absorption peaks at 2850-2918cm-1
could be assigned to the stretching vibration of CH3 and CH2, their
distortion vibration should be found in the region 1498~1377cm-1. The broad
peak at 3500cm-1 could be attributed to the characteristic absorption of OH in
the water crystal. The middle strong absorption peaks in 517cm-1 and 397cm-1
can be attributed to the stretching vibration of Zn-O(15). Some gas-lasers, such as HF,
HBr and HCl, have a strong absorption at about 500cm-1, so the two title
compounds can be researched as a new kind of low-power laser.
Table 2 Main Values of IR Spectra of Compounds 1 and 2(cm-1)
compounds |
1 |
2 |
uP-O |
1036 |
1059 |
uC-H |
2918,2850,1489,1466,1377 |
2918,2850,1468,1375 |
uC-O |
721 |
721 |
uO-H |
3500 |
3500 |
uZn-O |
517,397 |
521,390 |
3.3 NMR spectrum
For compound 2 as an example,dCH3=0.879ppm, dCH2=1.252ppm. The sharp peak appeared at 3.461ppm is the
characteristic peak of CH3-O, and the other peaks at the range of 3.4-4.1ppm
can attribute to the CH2 and CH link to O atom.
Expect the sharp peak at 3.461ppm of CH3-O, the spectrum of
compound 1 is very similar to the compound 2.
3.4 Mass spectrum
The mass spectrum analyses of zinc salts of phosphatidic acid are carried out for the
first time. Through the detection with computer, main ion fragments and their abundance
are listed in Table 3. For compound 1 as an example, the ion C3H7+(m/z
43, 100%) is the base peak. From the above table, we can easily explain the structure of
the compound. The serial arithmetical progression molecule ion peaks(C4H9+,
m/z 57, 75.52%; , C5H11+, m/z 71, 48.20%; C6H13+,
m/z 85, 26.30%; C7H15+, m/z 99, 9.67%; C9H19+,m/z
127, 6.48%; C18H37+, m/z 253, 10.00%) show the octadecyl
exists. Besides, we have obtained the fragments of PO4+(m/z 95,
6.85%), which supports the phosphatidic acid structure. The two peaks of Zn+(m/z
65, 2.91%) and ZnO+(m/z 81, 3.08%) can tell us the compound belongs to the zinc
salts.
Table 3 Main Fragments Ions of Compound 1 and 2
Compound 1 |
Compound 2 |
||||
Fragment ion |
m/z |
% |
Fragment ion |
m/z |
% |
C3H7+ |
43 |
100 |
C3H7+ |
43 |
100 |
C4H9+ |
57 |
75.52 |
C4H9+ |
57 |
62.52 |
C5H11+ |
71 |
48.20 |
C5H11+ |
71 |
34.93 |
C6H13+ |
85 |
26.30 |
C6H13+ |
85 |
15.25 |
C7H15+ |
99 |
9.67 |
C7H15+ |
99 |
27.40 |
C9H19+ |
127 |
6.48 |
C9H19+ |
127 |
no find |
C18H37+ |
253 |
10.00 |
C18H37+ |
253 |
10.64 |
PO4+ |
95 |
6.85 |
PO4+ |
95 |
11.21 |
Zn+ |
65 |
2.91 |
Zn+ |
65 |
2.91 |
ZnO+ |
81 |
3.08 |
ZnO+ |
81 |
4.45 |
CH3O+ |
31 |
no find |
|||
REFERENCES
[1] Ferid Murad, Angew Chem.Int.Ed.1999,38:1856.
[2] T. Kuiz, R.A. Wolf. and P.B.Corr. Circ.Res.1993,72:701.
[3] C.Pelassy, J.P. Breittamyer, D. Mary and C. Aussel. J.Lipid Medict.1991,4:199-209.
[4] D. English and G.S. Taylor Biochem. Biophys.Res.Commun.1999,175,423.
[5] P.G. Heyworth, U.G. Knaus, X. Xu, D.J. Uhlinger, L. Conroy, G.M. Bokoch and J.T.
Curnutte Mol.Biol.Cell 1993,4:261.
[6] T.H. Chuang, B.P. Bohl and G.M. Bokoch J.Biol.Chem.1993,268:26206.
[7] G.A. Senisterra, L.C. Van Gorkom and R.M. Epand
Biochem. and Biophys.Res.Commun.1993,190:33.
[8] S.B. Bocckino, P.B. Wilson and J.H. Exton Proc.Natl.Acad.Sci.USA.1991,88:6210.
[9] W.A. Khan, G.C. Blobe, A.L. Richards and Y.A. Hannun J.Biol.C.1994,268:9729.
[10] M. Billahm. Curr.Opin.Immunol,1993,5:114.
[11] J.H. Exton. Biochim.Biophys.Acta 1994,1212:26.
[12] R. Lauener, Yaping Shen, V. Duronio et al. Biochem. and Biophys, Res.Comman.1995,1:8.
[13] Ping Liu, Jiankai Cheng, Jian Wang. China. 01124699.5, 2001¡¡
¡¡