http://www.chemistrymag.org/cji/2003/059074ne.htm |
Sep. 1, 2003 Vol.5 No.9 P.74 Copyright |
(College of Chemistry & Environmental Science, Hebei University, Baoding 071002, China)
Received June 4, 2003; Supported by the National Science Pre-research Foundation of Hebei University, China (No.2003Q04)
Abstract A rapid and efficient synthesis
of 6-hydroxyl-2-phenylpyridazin-3-one in excellent yields from maleic anhydride with
phenylhydrazine hydrochloride under microwave irradiation is described. The effects of
different factors on this condensation reaction have been discussed.
Keywords microwave irradiation; synthesis; pyridazinone; condensation reaction
1 INTRODUCTION
There is considerable current interest in organic reactions under microwave
irradiation [1]. Some reactions that needed a long time to complete (i.e.
several hours or several days) could be carried out in several minutes under microwave
promotion [2,3]. Pyridazine-derivatives represent one of the most active
classes of compounds processing a wide spectrum of biological activity. They were widely
used in pharmaceuticals and agrochemicals [4,5]. Rohm-Haas Company had reported
that pyridazines exhibit useful plant growth regulating effects [6,7]. It was
reported that diacylhydrazines exhibit excellent insecticides [8-10]. Though
microwave irradiation its application has been applied to accelerate some condensation
reactions [11-13], in the synthesis of pyridazinone has not been
reported. Herein we wish to report a fast and efficient procedure to synthesize
pyridazinone under microwave irradiation. The results show microwave irradiation can
enhance this condensation reaction (Scheme 1).
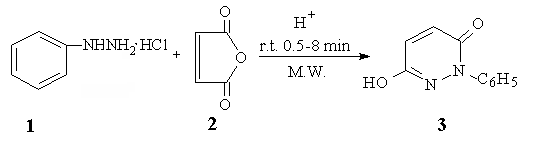
Scheme 1
2 EXPERIMENTAL
Melting points were uncorrected and were measured with micro-melting point apparatus.
IR spectra (KBr) were obtained on a Perkin-Elmer 983G spectrometer. 1H NMR
spectra were determined on a Bruker AC-80 spectrometer using CDCl3 as solvent
and tetramethylsilane (TMS) as internal reference. Mass spectra were determined on a
VG7070E spectrometer (EI, 70ev). Microwave irradiation was carried out with commercial
microwave oven (650W, 2450MHz). The products were characterized by 1H NMR
spectra and their melting points were compared with the literature values.
General procedure. A mixture of maleic anhydride 0.5883g (6.00mmol),
phenylhydrazine hydrochloride 0.4338g (3.00mmol) and dense hydrochloric acid 0.5ml (about
6.08mmol), in a cone bottle was introduced into the microwave oven and irradiated for
0.5-8min (output power at 100%). The progress of the reaction was monitored by TLC. Then,
H2O (10ml) was added to the reaction mixture and the solution's pH value was
adjusted to 7 with saturated Na2CO3. After cooling, the solution was
filtrated to give a white solid. The crude products were recrystallized from N,
N-dimethylformamide in 22.1-98.0% yields.
3 RESULTS AND DISCUSSION
This condensation reaction has been investigated at
various mole ratio of phenylhydrazine hydrochloride (1)
and maleic anhydride (2) when the power of microwave irradiation and the reaction
time were kept unchanged. The results were showed in Table 1.The yields were increased
with the mole ratio of reactant 1 and 2 up to 1:2 and then leveled off. When
the other reaction conditions were invariable, the effect of different microwave power on
the reaction was shown in Table 2. This may be due to the fact that
the more molecules absorbs microwave energy in short time the better the results are as
microwave radiation intensity increases. When the other reaction conditions were
invariable, the effect of different reaction time on the condensation reaction was shown
in Table 3. As summarized in T
Table 1 Changing the mole ratio of phenylhydrazine hydrochloride/ maleic anhydride
Entry |
Mole ratio a |
Power (%) |
Time (min) |
Yield (%) |
1 |
1:1 |
100 |
7 |
72.2 |
2 |
1:1.5 |
100 |
7 |
73.4 |
3 |
1:1.7 |
100 |
7 |
93.2 |
4 |
1:2 |
100 |
7 |
95.5 |
5 |
1:2.5 |
100 |
7 |
93.5 |
6 |
1:3 |
100 |
7 |
89.8 |
a The mole ratio is phenylhydrazine hydrochloride / maleic anhydride
Table 2 changing the power of microwave irradiation
Entry |
Mole ratio a |
Power (%) |
Time (min) |
Yield (%) |
1 |
1:1.7 |
100 |
8 |
93.0 |
2 |
1:1.7 |
50 |
8 |
82.9 |
3 |
1:1.7 |
10 |
8 |
79.2 |
4 |
1:2 |
100 |
8 |
93.8 |
5 |
1:2 |
50 |
8 |
85.7 |
6 |
1:2 |
10 |
8 |
80.1 |
Table 3 changing the reaction time of synthesizing pyridazinone
Entry |
Mole ratio a |
Power (%) |
Time (min) |
Yield (%) |
1 |
1:2 |
100 |
30s |
22.1 |
2 |
1:2 |
100 |
50s |
35.6 |
3 |
1:2 |
100 |
2min |
58.3 |
4 |
1:2 |
100 |
3min |
88.5 |
5 |
1:2 |
100 |
4min |
98.0 |
6 |
1:2 |
100 |
5min |
97.2 |
7 |
1:2 |
100 |
8min |
93.8 |
4 CONCLUSIONS
We concluded the best synthetical conditions of the pyridazinone were the mole ratio of
phenylhydrazine hydrochloride and maleic anhydride is 1:2, the power of microwave
irradiation was 100%(2450MHz), reaction time was 4 min., and the yield of pyridazinone was
up to 98.0%. In conclusion, a rapid and efficient method for the preparation of
6-hydroxyl-2-phenylpyridazin-3-one has been provided, which has the characteristics such
as its operational simplicity, high yields, short reaction time and low cost. This method
will be better than the existing one.
REFERENCES
[1] Micheal D, Mingos P, et al. Chem. Soc. Rev., 1991, 20: 1.
[2] Bram G, Majdoub A, et al. Tetrahedron, 1990, 46: 5167.
[3] Yuan Y C, Gao D B, et al. Synth. Commun., 1990, 20: 925.
[4] (a) Heinisch G, Koplent F H. Prog. Med. Chem., 1992, 29:141.
(b) Heinisch G, Koplent F H. Prog. Med. Chem., 1990, 27:1.
[5] Sha J J. Foreign New Pesticide Handbook. Beijing: Chemical Industry Press. 1993, 10:
130 (in Chinese).
[6] Fujimoto T T. (Rohm and Haas Company). Ger offen 2 808 795, 1978.
[7] Fujimoto T T. (Rohm and Haas Company). US 4 344 934, 1982.
[8] Hsu Adan Chi-Tung. (Rohm and Haas Company). EP 0 232 075, 1987.
[9] Hsu Adan Chi-Tung. (Rohm and Haas Company). EP 0 245 950, 1987.
[10] Hsu Adan Chi-Tung. (Rohm and Haas Company). EP 0 253 468, 1987.
[11] Li Z M, Zou X J, Yao E Y, et al. Yingyong Huaxue of China, 1993,10 (6): 86.
[12] Fan X J, You J M, Tan G Z, et al. Progress in. chem. 1998, 10 (3): 285.
[13] Li H Z, Zhang J S, et al. Synth. Commun., 2002, 32 (6): 927.
[14] Zhang X G. Fine organic compound technique handbook. 1. Beijing: Science and
Technology Press, 1992 (in Chinese).
กก
กก