http://www.chemistrymag.org/cji/2003/059075ne.htm |
Sep. 1, 2003 Vol.5 No.9 P.75 Copyright |
Li Hong, Ran Junguo#, Gou Li#
(Department of Chemistry, Jinan University, Guangzhou 510632, China, #Department of inorganic materials, Sichuan University, Chengdu 610065, China) Received Jul. 4, 2003.
Abstract The glass-ceramic mainly containing fluorophlogopite is one of widely used machinable ceramics. A new-type glass-ceramic containing fluorophlogopite has been synthesized in the K2O-CaO-MgO-Al2O3-SiO2-F system. Its crystalline was studied by XRD and EDS. The fluorophlogopite crystalline phase distinguishes from the family of fluoromica by K+ and Ca2+ acting as bonding ions altogether.
Keywords Fluorophlogopite, crystalline, glass ceramics .
1 INTRODUCTION
Glass ceramics are produced by controlled crystallization of appropriate glass. Mica-containing glass ceramic£¬ such as Dicor (Corning, Inc., Corning .NY)£¬ shows good machinability. The material can be machined to close tolerance with conventional methods. Because of its excellent biocompatibility and natural esthetics, the material is used as veneer the metal framework of crown, bridge, or inlay and onlay in restorative dentistry [1]. Application of the glass ceramic is limited, for its intrinsic mechanical brittleness leads to high clinic failure. Generally, the bonding strength of interlayer ions is the weakest in mica crystal, so the mechanical properties apparently are depended on the bonding strength of these interlayer ions. It has been reported, fluorophlogopite-type Ca- or Ba-mica [2,3], which Ca2+ or Ba2+ take place of alkali ions between interlayers, exhibits higher strength.
In the system, precipitation of fluorophlogopite-type Ca-mica with rod-shaped crystal from glass was formed. The composition and microstructure was studied with XRD, EDS and SEM.
2 EXPERIMENTAL
A bath mixture of nominal composition of SiO2 40%, Al2O3 12%,
MgO 10%, MgF2 24%, CaO 5%, K2O 1%, ZrO2 8% in weight
ratio was melt in a platinum crucible at 1550
3 RESULTS AND DISCUSSION
Figure 1 shows XRD pattern of the
glass-ceramic. Fluorophlogopite (mica) and t-ZrO2 are main crystalline phase.
The microstructure of the glass-ceramic (Figure 2) displays typical feature of the machinable
glass ceramic with rod like crystals isolated and interlocking. Tested by drilling and
dental CAD/CAM system, the glass-ceramic showed excellent machinability[4].
These rod-like crystals are 1-3
ZrO2 are most commonly used as nucleating agent in glass ceramic procedure. Some of ZrO2 dissolve in the glass system and Zr4+ substitutes one of Si4+ in [SiO4] tetrahedral, therefore EDS can detect in the rod like crystal (Figure 3). The others precipitate as t- or m-ZrO2 particles showed by XRD pattern (Figure 1).
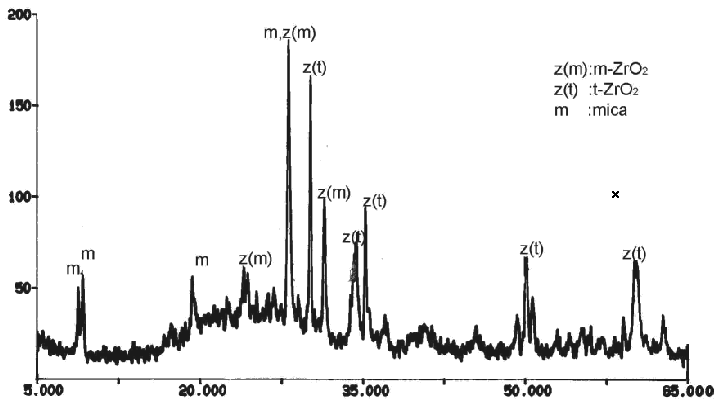
Fig.1 XRD pattern of the glass-ceramic

Fig.2 SEM of the glass ceramic
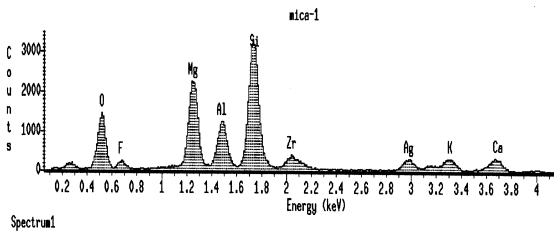
Fig.3 EDS pattern of the rod like
mica crystal, Ag is the coating material
4 CONCLUSION
In the K2O-CaO-MgO-Al2O3-SiO2-F system the
glass ceramic mainly containing a new-type fluorophlogopite has been synthesized. The
formula of the fluorophlogopite postulates K1-XCaX/2Mg3AlSi3O10F2.
K+ acts as bonding ion together with Ca2+.
[1] Thompson JY, Ideymann HO, Bayne C. J. Prosthet. Dent., 1996, 76(6): 129.
[2] Uno T, Kasuga T, and Makajima K. J. Am. Ceram. Soc., 1991, 74(12): 3139.
[3] Uno T, Kasuga T, and Makajima K.J, et al. J. Am. Ceram. Soc., 1993, 76(6): 539.
[4] Li H. Doctoral dissertation. Sichuan University, Chengdu, Sichuan. 2002.
[5] Mazurine OV. Phase separation of glass, New York: North-Holland, 1984: 35. ¡¡