http://www.chemistrymag.org/cji/2003/05a076ne.htm |
Oct. 1, 2003 Vol.5 No.10 P.76 Copyright |
Liu Lu, Wu Qingsheng, Ding Yaping#, Liu
Huajie
(Department of Chemistry, Tongji University,
Shanghai, 200092; #Department of Chemistry, Shanghai University,
Shanghai 200436, China)
Received Jul.15,2003; Supported by the National Natural Science Foundation of China (20071025) and Nano-foundation of Shanghai(0259nm021; 0114nm004)
Abstract A novel biomimetic emulsion liquid membrane system with carrier has been developed to the synthesis of SnO2 nanoparticles. This membrane system consists of kerosene as solvent, L152 as surfactant, TBP as carrier, SnCl4 solution as external aqueous phase and NH3·H2O-NH4Cl (pH=10) buffer solution as internal aqueous phase. The precursor Sn(OH)4 nanoparticles with diameter of 3-4 nm are initially synthesized through ion-transport between this membrane. The final product SnO2 nanoparticles with diameter of 2-3 nm are prepared after treating precursor at 400°C for 6h. Transmission electron microscopy (TEM) and X-ray diffraction (XRD) are used to characterize the product. Ultraviolet-visible (UV-Vis) spectrum shows a blueshift of 20 nm while comparing with that of bulk materials, which can be attributed to the quantum size effect of nanomaterials.Keywords emulsion liquid membrane, SnO2, nanoparticles, biomimetic synthesis 1. INTRODUCTION
Tin dioxide is one of the most important metal oxide semiconductor materials, and is extensively applied to ceramic, gas sensor and catalyst [1-3], etc. Especially, scientists are interested in SnO2 nanomaterials for their evident surface effect and pay much attention to their synthesis methods[4-6]. There are several methods to be used for obtaining SnO2 nanomaterials such as surfactant-mediated method[7,8], Sol-Gel[9], chemical precipitation method[10], hydrolytic method[11,12], hydrothermal method[13-14], etc. However, in order to avoid agglomeration and obtain smaller SnO2 nanoparticles in size, many chemists and material chemists have made efforts to research on more efficient synthesis methods.
It is well known that cell has the functions of ATP-driven active transport[15] and forming nanosize biomineralization products using collagen as template[16]. For example, many biomaterials like tooth and bone consist of nanocrystals assembled through bio-macromolecule as template[17]. So it is feasible that nanomaterials can be synthesized by simulating the bio-process. In this paper, a novel biomimetic emulsion liquid membrane system with carrier is developed to the synthesis of SnO2 nanoparticles. 2. EXPERIMENTAL
The water-in-oil (w/o) emulsion was prepared in emulsification step by initially dissolving the surfactant dialkylene succinimide (L152) and tributyl phosphate(TBP) in the kerosene and then adding NH3·H2O-NH4Cl (pH=10) buffer solution, The emulsification was carried out for 10-15 minutes when the agitator was run at the rate of 3000 rpm. The volume ratio of membrane phase to internal aqueous phase (Roi) was 1.0.
According to certain Rew (volume ratio of external aqueous phase to w/o emulsion), 25ml w/o emulsion prepared above was added to 50ml external aqueous phase (0.1M SnCl4 solution). Then the w/o/w emulsion was agitated at 500rpm for 10min. The w/o emulsion and SnCl4 external aqueous phase solution were separated by funnel, w/o emulsion was centrifugated, and the precipitate was washed with petroleum ether and alcohol for 3-4 times. The precipitate was treated at 400°C for 6 hours, then the sample was immersed into alcohol. 3. RESULTS AND DISCUSSION
Figure 1(a) shows the morphology and structure of SnO2 sample (Philips EM400ST transmission electron microscopy, accelerating voltage of 100 kV), TEM image reveals both smooth solid particles structure with diameters of 2-3nm. TEM image of precursor Sn(OH)4 shows in Figure 1(c) and reveals both smooth and amorphous solid particles structure with diameters of 3-4nm. On the other hand, the SnO2 particle size was not changed while increasing the temperature of precursor treatment, Figure 1(b) reveals TEM image of SnO2 sample with diameter of 2-3nm which comes from precursor treated at 300°C.
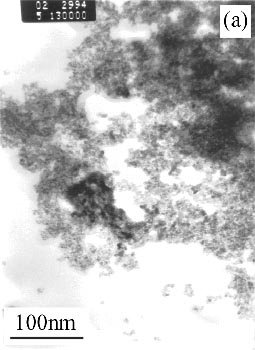
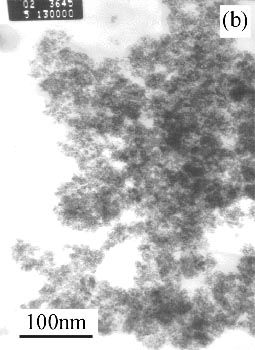
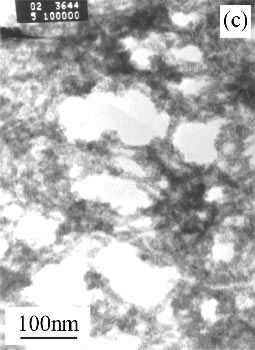
Figure 1 Morphology of SnO2 sample and Sn(OH)4 precursor
(a) SnO2 products at 400°C; (b) SnO2 products at 300°C; (c) Sn(OH)4 precursor
A XRD pattern of the as-prepared SnO2 sample is shown in Figure 2 (XRD, Philips Pw1700 X-ray diffractometer, employing Cu-Kα radiation, λ=1.54056
Å), all peaks in the pattern can be identified to the known cubic structure of SnO2 (JCPDS 21-340). The measured lattice parameter is a=6.068Å, consistent with the reported value (a=6.077Å).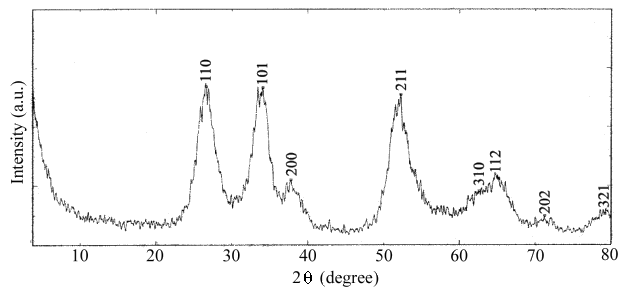
Figure 2 XRD pattern of SnO2 nanoparticles
In the synthesis process,
the choice of reagent in internal-aqueous phase is important to obtain pure SnO2,
alkali with Sn4+ form precursor Sn(OH)4, however, only using base NH3·H2O
as reagent in internal-aqueous phase does not bring about impurity(salt) after the
precursor is treated at high temperature. When NH3·H2O
concentration is too high, the emulsion liquid membrane system in SnCl4
external-aqueous phase solution(pH<1) unsteadily exist, on the other hand the forming
precursor is influenced if NH3·H2O concentration is low, so
NH3·H2O-NH4Cl buffer solution (pH10) is chosen as
reagent in internal-aqueous phase which have pH value steady and small size precursor
formed. At the same time, formed NH4Cl is decomposed in the form of NH3
and HCl which evaporate, so pure SnO2 nanoproducts are obtained. Tributyl
phosphate is the carrier in the system and transportation process is shown in Figure 3.
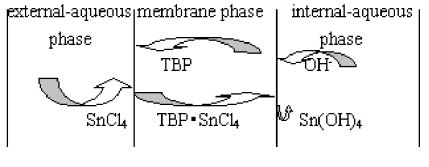
Figure 3 Transportation process of SnCl4
Precursor Sn(OH)4
could be formed as follows: Combine Sn4+ in external-aqueous phase with TBP in
membrane phase to form associating compounds on interface between external-aqueous phase
and membrane phase. According to concentration gradient in membrane phase, diffusion of
concentration happens. Then these associating compounds arrive at interface between
membrane phase and internal-aqueous phase and react with OH- in
internal-aqueous phase to form Sn(OH)4 nucleus. Because there are carriers(TBP
concentration 10%), transportation speed is speeded up; and because Ksp[Sn(OH)4]
is small(10-56), congregate precipitate rate is far larger than that of
orientation. So a great many of Sn(OH)4 nucleus are formed and rapidly
precipitated in the form of amorphous Sn(OH)4 nanoparticles.
Figure 4 shows the absorption peak of ultraviolet-visible absorption,
the graph reveals there is an absorption peak in 324 nm to the SnO2
nanomaterials. It is well known that band gap energy of SnO2 bulk materials is
3.6eV, the most peak of SnO2 nanoparticles(λ=324nm) has a large deviation(20 nm), which is compared with the
most peak of SnO2 (λ=344nm). The reason is
that as the particle size decreases, the blueshift occurs from the bulk band gap of 3.6eV
(344nm) due to the well-known quantum size effect.
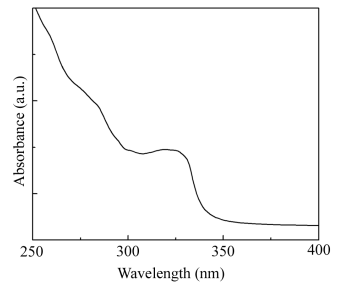
Figure 4 Ultraviolet-visible absorption of SnO2 samples REFERENCES
[1] Torvela H, Leppavuori S. Inte. High. Technol. Ceram., 1987, 3: 309.
[2] Harrison P G, Willet M J. Nature, 1988, 332: 337.
[3] Stampfl S R, Chen Y, Dumesis N et al. J. Catal., 1987, 105: 445.
[4] Ocana M, Serna C J, Matijevic E. Mater. Lett., 1991, 12: 32.
[5] Matije E. Ann. Rev. Mater. Sci., 1985, 15: 483.
[6] Ocana M, Matijevic E. J. Mater. Res., 1990, 5: 1083.
[7] Wang Y D, Ma C L, Sun X D, Li H D. Nanotechnology, 2002, 13(5): 565.
[8] Wang Y D, Ma C L, Sun X D, Li H D. Inorg. Chem. Commun., 2002, 5(10): 751.
[9] Quaranta F, Rella R, Siciliano R et al. Sensors & Actuators B-Chemical, 2002, 84(1): 55.
[10] Yao M Q, Wei Y H, Hu L Q et al. Transactions of Nonferrous Metals Society of China, 2002, 12(5): 833.
[11] Deng Z X, Wang C, Li Y D. J. Am. Ceram. Soc., 2002, 85: 2837.
[12] Monedon S, Cellot A, Ribot F et al. J. Mater. Chem., 2002, 12(8): 2396.
[13] Wang C Y, Hu Y, Qian Y T. Nanosructured Mater., 1996, 7(4): 421.
[14] He Y P, Li Y D, Yu J et al. Mater. Lett., 1999, 40: 23.
[15] Voet D, Voet J G. Biochemistry. John Wiley & Sons, Inc.: New York, 1995.
[16] Mann S. J. Mater. Chem., 1995, 5: 935.
[17] Wu Q S, Zheng N W, Li Y D et al. J. Membr. Sci., 2000, 172: 199.
载体乳化液膜法仿生合成二氧化锡纳米粒子
刘璐 吴庆生 丁亚平# 柳华杰
(同济大学化学系 上海 200092; #上海大学化学系 上海 200436)
摘要 本文提出并设计了新型的载体乳化液膜体系来仿生合成SnO2纳米粒子. 在煤油-L152-TBP体系中, 以SnCl4溶液为外相, NH3·H2O-NH4Cl (pH=10)缓冲液为内相, 通过离子传输, 首先合成了直径3-4纳米的前驱物Sn(OH)4纳米粒子. 然后将前驱物在400°C热处理6小时, 得到了直径2-3纳米的SnO2纳米粒子. 使用透射电子显微镜(TEM)和X射线粉末衍射(XRD)对产物形貌和结构进行了表征. 紫外-可见吸收光谱(UV-Vis)表明产物的吸收光谱对于块体材料产生了20纳米的蓝移, 表明了纳米粒子的量子尺寸效应.
关键词 乳化液膜 二氧化锡 纳米粒子 仿生合成