http://www.chemistrymag.org/cji/2003/05b084ne.htm |
Nov. 1, 2003 Vol.5 No.11 P.84 Copyright |
Ren Yixiaa, b, Chen Peic
, Chen Sanpinga, Zhao Fengqia, c ,Yang Xuwua, Gao Shenglia
(a Shaanxi Key Laboratory of Physico-Inorganic Chemistry, Department of
Chemistry, Northwest University, Xi'an 710069; b College of Chemistry and
Chemical Engineering, Yan'an University, Yan'an 716000; c Xi'an Modern
Chemical Institute, Xi'an 710072)
Received Jun.16, 2003; Supported by NSFC (No.20171036) and NSF of Shaanxi Province (No.2002B03)
Abstract The complex of ZnAc2·2H2O
and Valine was prepared in the mixture solvent of water - acetone, the composition of
which was identified as Zn(Val)Ac2 by chemical and elemental analyses. The
bonding characteristic and thermo stability of the title complex were investigated by IR,
DSC and TG-DTG, its enthalpies of decomposition were obtained as 21.50 J·g ¨C1and 376.60 J·g -1. The constant volume
combustion energy of the complex, ![]() , was
determined as (-5698.87¡À7.89 )kJ·mol-1 by a precise rotating-bomb
calorimeter at 298.15 K. Standard enthalpy of combustion,
, was
determined as (-5698.87¡À7.89 )kJ·mol-1 by a precise rotating-bomb
calorimeter at 298.15 K. Standard enthalpy of combustion,![]() , and standard enthalpy of formation,
, and standard enthalpy of formation, ![]() , for the complex were calculated as (-5701.97¡À7.89)
kJ·mol-1 and (-619.64¡À2.59)kJ·mol-1, respectively.
, for the complex were calculated as (-5701.97¡À7.89)
kJ·mol-1 and (-619.64¡À2.59)kJ·mol-1, respectively.
Keywords ZnAc2·2H2O, Valine, Zn(Val)Ac2,
standard molar enthalpy of formation
The complexes of zinc with L-a-amino acids as additives have been
widely applied in medicine, foodstuff and cosmetics [1-3]. The preparation
methods of making zinc amino acids have been comprehensively reviewed [4-5].
The solubility properties of ZnAc2-Val-H2O [Val = Valine (C5H11O2N)]
system at 298.15 K has been investigated by the semimicro-phase equilibrium method [6].
The phase diagram is a simple one, in which the phase region of the complexes does not
exist. The preparation of the crystal of Zn(Thr)SO4·H2O was
reported in literature [7] : to the reaction liquid of ZnSO4 and L-a-Thr with the
molar ratio of 1:1, three times volume acetone was added, and the product was obtained.
In this paper, the solid complex of Zn(Val)Ac2 was prepared
in the mixture solvent of water-acetone ratio of 1:10. The composition of the complex was
identified as Zn(Val)Ac2 by chemical and elemental analyses. The bonding
characteristic and thermo stability of the title complex were investigated by IR, DSC and
TG ¨C DTG. Its enthalpies of decomposition were
obtained from the DSC curve. The constant-volume combustion energy of the compound was
determined by a precise rotating-bomb calorimeter at 298.15 K; its standard enthalpies of
combustion and standard enthalpies of formation were calculated.
1 EXPERIMENTAL
1.1 Reagents and equipments
ZnAc2 · 2H2O, A.
R. (Xi'an Chemical Company); L-a-Val, B. R. (Shanghai Kangda Company), purity > 99.95%;
acetone, A. R. (Xi'an Chemical Company), the others are of A. R. grade. Zn2+ was
determined with EDTA by complexometric; Val by formalin' method, the
Zn2+ was removed by precipitating with K2C2O4 before
it was titrated. Carbon, hydrogen and nitrogen analyses were carried out on a 2400 type
elemental analyzer of PE Company, DSC experiment was performed with a Model CDR-1 thermal
analyzer made in the Shanghai Balance Instrument Factory. TG-DTG curve were recorded on a
TG-7 type thermobalance under the heating rate of 10ºC·min-1 and
the flow rate of N2 of 60 mL · min-1, the sample weight is
about 1mg. IR analyses were carried out by a BEQ, UZNDX-550 IR spectrophotometer with KBr
pellet. The constant-volume combustion energy of compound was determined by a precise
rotating-bomb calorimeter ( RBC-type ) [8].The experimental method and steps
were the same as the literature [9].The initial temperature was regulated to
(25.0000 ¡À0.0005)ºC by a super
constant temperature thermostat and the initial oxygen pressure was 2.5 MPa. The final
products were analyzed by method in literature [9]. The correct value of the heat exchange
was calculated according to the Linio-Pyfengdelel-Wsava formula [10]. The
calorimeter was calibrated by benzoic acid of 99.999% purity from Chengdu Chemical reagent
Factory. Benzoic acid had an isothermal heat of combustion at 25 ºC of (-26434 ¡À 5.80) J·g-1. The energy equivalent
of calorimeter was (17936.01 ¡À 9.08) kJ·K-1. The precision was 5.06¡Á10-4.
1.2 Preparation of complex
ZnAc2·2H2O and L-a-Val with the molar
ratio of 1:1 were dissolved in an appropriate amount of water, kept the reaction at 60-70ºC for 8 hours, and allowed to cool. To the solution,
10 times volume acetone was added, and large amount white precipitate was obtained. The
precipitate was filtered off, washed with acetone, and kept in vacuum over P4O10
to dryness for being used. The yield is 33 % (Table 1). Anal. Calc. for Zn(Val)Ac2 :
Zn2+ 21.71, Val 38.97, C 35.96, H 5.70 , N 4.66 ; found Zn2+ 21.78,
Val 38.56, C 36.01, H 5.80, N 4.68.
Table1 Experimental results of the preparation of the complex with different volume ratios of water and acetone
| Volume ratio | 1:3 |
1:5 |
1:10 |
1:15 |
1:20 |
1:25 |
| Phenomenon | turbid |
turbid |
precipitate |
precipitate |
precipitate decreasing gradually |
|
| Yield (%) | --- |
17 |
33 |
32 |
26 |
22 |
2.1 IR spectroscopy
Data of IR absorption for main groups of ligand and complex are listed in Table 2. Non-existing the characteristic absorption bands of -COOH group at 1700-1750 cm-1 for complex reveals that Val still keeps zwitter-ion structure [11]; wide shifts of n
Table 2 Data of IR absorption for main groups of ligand and complex (©M-1)
Sample |
nNH3as | nNH3s | dNH3as | dNH3s | nCOO-as | nCOO-s |
Ligand |
3454.6 |
2976.9 |
1614.9 |
1506.3 |
1506.3 |
1425.5 |
| Complex | 3331.0 |
2962.0 |
1615.0 |
1510.0 |
1466.0 |
1394.0 |
The TG-DTG curves of the complex are showed in Fig.1. On the basis of the thermal decomposition results (calculated values) of the complex, the thermal decomposition mechanism can be postulated as follows (the middle is the peak temperatures of DTG ):
From the curves, it can be seen the mass residues of every stage of the complex are rather close to the calculated values. The DTG curve shows the thermal decomposition process of Zn(Val)Ac2 can be divided into two stages. In the first stage, the skeleton of the complex is partly broken and decomposed into ZnO. The characteristic absorption peaks of the complex and ZnO (356 cm-1) are observed in the spectra of decomposition products. In the second stage, the complex is completely decomposed into ZnO. Its IR spectrum coincides with the standard IR spectrum of ZnO. (Fig.2).
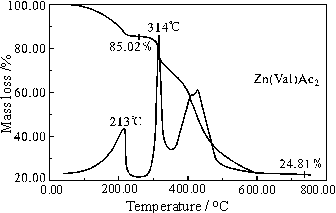
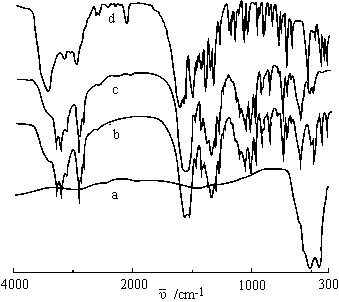
Figure 1 TG-DTG curve for Zn(Val)Ac2 Figure 2 IR spectra of ligand and its metal compounds
a. ZnO, b. 4Zn(Val)Ac2·ZnO, c. Zn(Val)Ac2, d. Val
The DSC curve of the complex is showed
in Fig.3. Their decomposition temperatures coincide basically with the TG-DTG
thermal-analytical ones. The two peaks in the figure can be considered as the enthalpy
changes of the decomposition of the complex. As shown in the figure, ![]() = 21.50 J·g -1,
= 21.50 J·g -1, ![]() = 376.60 J·g ¨C1.
= 376.60 J·g ¨C1.
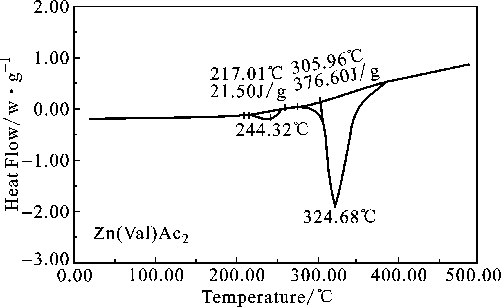
Figure 3 DSC curve for Zn(Val)Ac2
Table 3 The experimental results of constant-volume combustion energy for Zn(Val)Ac2
No. |
m/g |
Calibrated
heat of combustion wire |
Calibrated
heat of acid |
Calibrated ¦¤T/K |
Combustion
energy of sample |
1 |
1.00794 |
10.80 |
32.49 |
1.0620 |
18923.21 |
2 |
1.07598 |
11.70 |
34.55 |
1.1371 |
18980.24 |
3 |
1.04148 |
12.60 |
33.50 |
1.0994 |
18957.52 |
4 |
1.05243 |
9.90 |
33.74 |
1.1101 |
18945.63 |
5 |
1.05965 |
12.60 |
33.97 |
1.1196 |
18975.16 |
6 |
1.00230 |
12.60 |
32.13 |
1.0575 |
18947.44 |
Mean |
18955.81¡À7.89 |
The experimental data of constant volume combustion energy of Zn(Val)Ac2 are showed in Table 3.
The standard enthalpy of combustion,
The standard enthalpy of formation of the complex,
where£¬
The standard mole enthalpy of formation is calculated as£¨-619.64 ¡À 2.59 £©kJ·mol-1.
REFERENCES
[1] Mahmoud M, Abdel-monem S, Paul M. US Patent 4 039 681, 1977-08-02.
[2] Taguchi S, Inokuchi M, Nakajima N. WO Patent 10 178, 1992-06-25.
[3] Harvey H, Ashmed , Kaysville utah. US Patent 4 830 716, 1989-05-16.
[4] Gao S L, Liu J R, Ji M, Yang X W, et al. Chinese Sci. Bull. (Kexue Tongbao), 1998, 43
(14): 1496.
[5] Gao S L, Hou Y D, Liu J R,et al. Chinese Chem. Bull.(Huaxue Tongbao), 1999, 11:30 .
[6] Guo L J, Zhang F X, Tang X Q, et al. Journal of Northwest University (Natural Science
Edition), (Xibei Daxue Xuebao) 2002, 23(1): 29.
[7] Gao S L, Zhang X Y,Yang X W, et al. Acta Chmica Sinica (Huaxue Xuebao), 2001, 59 (1):
73.
[8] Gao S L, Chen S P, Yang X W,et al. Chin. J. Chem.(Zhongguo Huaxue), 2001,19 (11):
1037.
[9] Yang X F, Yang X W, Sun L Z,et al. Chem.J. Chin, Univ.(Gaodeng Xuexiao Huaxue Xuebao),
1986, 7: 363.
[10] Popov M M, Thermometry and Calorimetry, Moscow University Press Moscow, 1954,382.
[11] Niu C J, Zhang S G, Wang Z L, et al. Chem. J. Chin. Univ. (Gaodeng Xuexiao Huaxue
Xuebao), 1991, 12 (10): 1386.
[12] Cox J. D. J. Chem. Thermodyn. 1978, 10: 903.