http://www.chemistrymag.org/cji/2003/05b085pe.htm |
Nov. 1, 2003 Vol.5 No.11 P.85 Copyright |
Study on the reaction rate and mechanism of synthesis of tert-butyl ethyl ether in liquid phase based on synergetics
Yao Ruiqing, Yang Bolun
(Chemical Engineering Department of Xi'an Jiaotong University, Xi'an 710049, China)
Abstract Synergetics is employed as a
guideline to establish a new method for researching the kinetics of the complex chemical
reaction. The difficulties in applying the synergetics to chemical kinetics were
eliminated by introducing a non-singular linear transformation to convert the rate
equations into the Haken's form. The expressions of the order parameter and its evolution
in time can be analytically obtained. The mechanism of synthesis of tert-butyl ethyl ether
(ETBE) using alcohol-alcohol in liquid phase is discussed to demonstrate the advantages of
this new method. The rate equations were obtained which agree well with the experiments.
It is shown that the synergetics approach presented here offers a powerful means to solve
rate equations analytically for a complex reaction, therefore, it is no need of the
application of steady-state approximation that is frequently used in chemical kinetics.
This method is proved to have a wide range of validity in the research of the chemical
kinetics.
Keywords tert-butyl ethyl ether (ETBE), synthesis mechanism, synergetics
Generally, most chemical reaction processes are in the state of non-equilibrium [1]. Therefore, the dynamic processes of chemical reaction should belong to the category of non-equilibrium statistical mechanics. The latest theory for researching such a non-steady state is synergetics presented by H.Haken [2, 3]. In synergetics, Haken described the behavior of systems that was near the critical point and elaborated the conception of slaving principle and order parameter. Synergetics considered that the evolution of a system is controlled by the order parameter. The final organization and the degree of order are decided by the order parameter. The physical meaning of the order parameter is deferent in different systems. For example, in laser system, the intensity of light field is order parameter; in chemical reaction, the concentration or the number of particles is chosen as order parameter. In this theory, Haken induced the fundamental evolution rule of the non-equilibrium systems and the most important is that it makes people describe the disorder-order transition of different systems with the same mathematical model. But it is difficult to solve the equation set of reaction rate with Haken's methods directly. It is a reason that why the adiabatic approximate method have not been used in the field of kinetics of chemical reaction [4].
In this paper, a specified reaction rate equation is converted to Haken's form by using a non-singular linear transformation about kinetics variable directly and then the difficulty that involved in the employment of synergetics can be avoided. Thus, it is unnecessary to apply steady-state approximation that is frequently used in the investigation of chemical kinetics. With this method, the mechanism of synthesis of tert-butyl ethyl ether in the liquid phase [5, 6] was analyzed; the rate equation set was solved and the analytic expressions of order parameters and other variables were obtained. The calculated reaction rate compared favorably with the experimental data.
2 EXPERIMENTAL SECTION
2.1 Materials
Ethanol of analytical grade and tert-butyl alcohol of chemical grade were purchased
from Xi'an Chemical Reagent Factory and Shanghai Chemical Regent Factory respectively.
tert-butyl ethyl ether was provided by Tokyo Kasei. Catalyst was merchant ion exchange
resin S-54.
2.2 Apparatus and procedure
The reactor consisted of a three-necked flat-bottomed flask of 2¡Á10-4m3
capacity fitted with a condenser in the central opening, a sampling device and a
thermowell. The reaction temperature was controlled by a water bath. The mixture was
magnetically stirred at about 600 rpm in all the runs. It was found in the preparatory
experiment that the reaction rate was independent of agitation speeds in the range of
100-800rpm and the particle size did not influence the reaction rate, which showed that
the external and internal diffusion resistance were not significant. Therefore, the
internal and external diffusion could be ignored and the process was exclusively
controlled by reaction kinetics.
The concentration of each component was detected in a gas
chromatograph, with a 4 m column of Gaskuropack 54 as packing material at 463 K using a
TCD detector. Helium at 0.12 Mpa was used as the carrier gas. Separation had been achieved
for all the components.
3 ANALYSIS OF THE REACTION KINETICS
In order to elucidate the application routine of the method, the reaction kinetics of
the synthesis of ethyl tert-butyl ether in liquid phase were analyzed. The mechanism is
shown as follows:
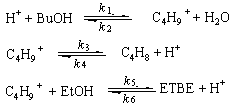
In this process, tert-butyl alcohol is
catalyzed to be carbocation free radical. Then, the carbocation combine with ethanol to
form ETBE; meanwhile carbocation is decomposed to isobutene. If C1=CBuOH,
C2=CC4H9+, C3=CH2O,
C4=CC4H8, C5=CEtOH,
C6=CETBE express the concentration of each component
then, the rate equation set that related to the previous stated mechanism will be written
as the following:
![]() 1 = -k1C1 + h1
(3.1)
1 = -k1C1 + h1
(3.1)
![]() 2= k 1C1 ¨Ck 3C2 + k 4C4
+ k 6C6 + h2
2= k 1C1 ¨Ck 3C2 + k 4C4
+ k 6C6 + h2
Here, ri represents reaction rate of each reaction, hi represents the non-linear part of each reaction rate equation. As catalyst, the concentration of H+ is high enough, and it is considered to be constant at any stage of the reaction.
According to the known reaction mechanism of the above process [6], the production of C4H9+ from C4H8 can be ignored, the k 4 in the above equation sets then become zero and h1= k 2C2C3, h2= - k 2 C2 C3- k5 C2 C5, h3= - k2 C2 C3, h4=0, h5= - k5 C2 C5, h6= k5 C2 C5.
The boundary condition is:
C1(0)=A, C5(0)=B, Ci(0)=0 (i=2, 3, 4, 6)
Equation (3.4) is not independent, only equations (3.1) (3.2) (3.3) (3.5) (3.6) need to be solved.
4 PRINCIPLE OF THE
NON-SINGULAR LINEAR TRANSFORMATION
In order to obtain the equations of order parameters by using adiabatic approximate
method, the most important step is to find the order parameters. Haken suggested a method
to find the order parameters by distinguishing the changing rate in time of state
variables, which is a distinctive and effective method. With the method, many variables
can be represented by only one or a few ones of them. Then, it becomes possible to
research their effect on the evolution of system in mathematics.
Because the rate equation set of chemical reaction generally contains
linear and non-linear part, the linear transformation can be used to convert it into
Haken's form instead of using the method of variation parameters used by Haken.
Suppose that the rate equation set is in the form as follows (the
concentrations are dimensionless in the following discussion):
![]() =AC+h (C1,
C2, ..., Cn)
=AC+h (C1,
C2, ..., Cn)
where,
Suppose
C =VC' or C'=V- 1C (4.2)
whereV is a matrix of n¡Án with vij (i, j=1,2,...,,n) as its element (it has inverse matrix V- 1) and C'represents a column with Cj' as its component. Substituting (4.2) into (4.1), the following relational expressions are gotten:
where£è'(C') represents the transformed h (C). And if
V-1A V=¡²l1,l2, ..., l£î¡³ (4.4)
where¡²l1£¬l2£¬¡l£î¡³is diagonal matrix,lj (j=1, 2, ..., n) is matrix element, equation(4.3) can be turned to the following form:
Thus equation (4.5) is obtained as the Haken's form of equation(4.1). Therefore the above method can be considered as the process of finding the matrix element (Vij) of the non-singular matrix V and the value of lj. Since the equation set (4.4) can be written as the following form:
the method can be further considered as the process of finding the characteristic value, lk of equation (4.6) .
5 DERIVATION OF ORDER PARAMETERS EQUATIONS
The order parameter equations could be derived based on the above principle. The C matrix
and coefficient matrix A of equation set are (5.1).
A£½ 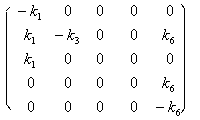 C£½
C£½ ![]()
The characteristic equation of matrix A is:
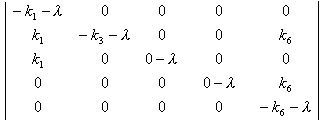 =0
(5.2)
=0
(5.2) The solution of this equation is:
l1£½-k1, l2= -k3, l3=l5= 0, l6= -k6 (5.3)
The equation set corresponding to (4.6) is:
( - k1 -lK)V1£Ë= 0
k1V1£Ë+(-k 3-l£Ë)V2£Ë+ k6V6£Ë=0
k1V1£Ë+(-l£Ë)V3£Ë=0 (5.4)
(-l£Ë)V5£Ë+ k 6V6£Ë=0
(-k6-l£Ë) V6£Ë=0
The following solution can be obtained from (5.3) and (5.4) with the orthonormal relation condition:
V11=
V33=V53=
Matrix V can be written as the following form:
V=
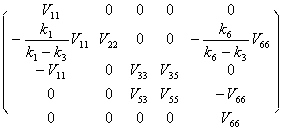 (5.5)
(5.5)And then
V-1=
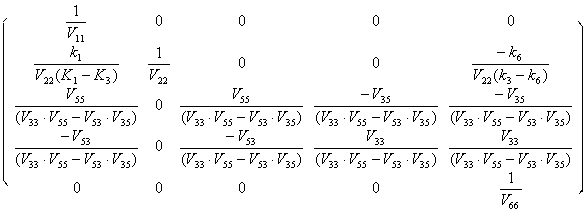 (5.6)
(5.6)Therefore, the relationship corresponding with (4.2) becomes:
 (5.7)
(5.7)With the transformation of (5.7), (3.1)-(3.6) can be written as follows:
Where, h1' =
h3'=
h5'=
h6'=
In the equation set (5.8), the variables
C1£½A e£k1At (5.9)
C2£½k1/ k2(A£1)[1/2£1/(1+e k1At)] (5.10)
C3=A£Ae- k1At (5.11)
C4£½(A£1)k3/ k2[(-k 1/2)t£«(1/A)ln(1£«e k1At)] (5.12)
C5=e(M/2- k6)t (1+e k1At)M/ k1A£2 k5/ k2(1-1/A) (5.13)
According to the material balance of the reaction:
C6=B-C5 (5.14)
Thus, as shown in (5.9) - (5.14), the expressions of the change of the concentration in time in the reaction process are obtained. 6 RESULTS AND DISCUSSION
When a chemical reaction reached equilibrium, the rate of creation of free radicals or chain carrier is equal to the rate of consuming, namely that the variation rate of concentration with time is zero. If this reaction was researched by using steady state approximation, it must be provided that
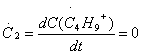 .
But this is improper when the reaction is in the beginning phase or far from equilibrium
state. This hypothesis is needless when the advantages of the adiabatic approximate method
in the principle of synergetics were taken.
.
But this is improper when the reaction is in the beginning phase or far from equilibrium
state. This hypothesis is needless when the advantages of the adiabatic approximate method
in the principle of synergetics were taken.The reaction rate constants as the function of temperature were also determined to be the following:
Fig.1 and Fig.2 are the relations of concentration to time. Reaction temperature is 328K. The lines in Fig.1 are the calculated results with the genetics algorithm (GA) by using the results of synergetics method, the lines in Fig.2 shows the relations of concentration to time calculated with GA by using the results of Runge-Kutta as in the earlier article [7]. It can be seen that the results of Runge-Kutta are only slightly different to the results of synergetics method: the results of Runge-Kutta are a little lower than experiment data while the results of synergetics are a little higher. Fig.3 and Fig.4 are the relations of concentration to time. Reaction temperature is 318K. The fitted results of synergetics are better than that of Runge-Kutta. The method of Runge-Kutta is a kind of relatively precise method and the synergetics is an approximate method, but the results are similar to each other. Thus, the validity of the method based on the synergetics is proved.
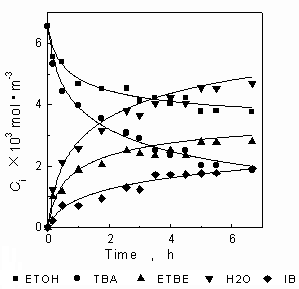
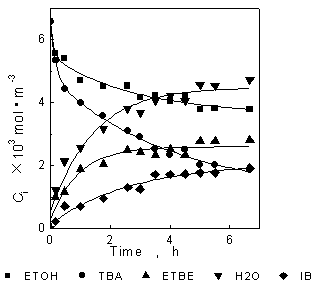
Fig.1 Relations of concentration to
time
Fig.2 Relations of concentration to time
--experiment data ¡ªcalculation(synergetics)
--experiment data ¡ªcalculation(R-K)
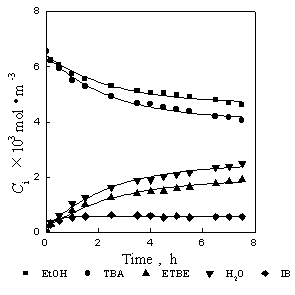
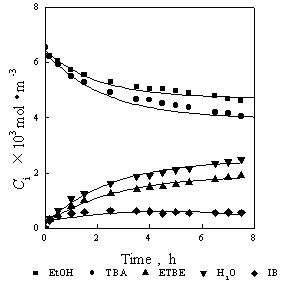
Fig.3 Relations of concentration to time
Fig.4 Relations of concentration to time
--experiment data ¡ªcalculation(synergetics)
--experiment data ¡ªcalculation(R-K)
Hereby, in order to deal with the complicated chemical kinetics problems the principles of the synergetics can be employed as: transform the certain equation set of chemical kinetics into Haken's form directly by introducing a non-singular linear transformation and then solve them by taking advantage of the adiabatic approximate method to obtain the expression of the evolution of order parameter in time. For the synthesis of tert-butyl ethyl ether, the result of the method based on the synergetics agreed well with the previous experimental data. It shows that the synergetics provide an effective algebraic technique¡ªnon-singular linear transformation that can be used to solve the complex rate equation set of chemical kinetics.
REFERENCES
[1] Zhao X Z. Principles of the kinetics of chemical reaction (Huaxue Fanying
Donglixue Yuanli). Beijing: High Education Press, 1984.
[2] Haken H, translated by Guo Z A. Advanced synergetics (GaoDeng Xietong Xue). Beijing:
Science Press, 1989.
[3] Wu D J, Cao L, Chen L H. Principles and application of synergetics (XietongXue Yuanli
Ji Yingyong). Wuhan: Huazhong university of science and technology Press, 1990.
[4] Guan D R, Yi X Z, Ding S L. Journal of Molecular Science (Fenzi Kexue Xuebao), 1998,
14(1): 13.
[5] Yang B L, Yang S B, Yao R Q. React. Funct. Polym., 2000, 44 (2): 167.
[6] Christine H. Ind. Eng. Chem. Res., 1995, 34: 3784.
[7] Yang S B, Yang B L. Journal of Chemical Industry
and Engineering (Huagong Xuebao), 2002, 53(1): 54.
¡¡
¡¡ ¡¡