http://www.chemistrymag.org/cji/2003/05b086pe.htm |
Nov. 1, 2003 Vol.5 No.11 P.86 Copyright |
Microwave synthesis of Ba1-xSrxTiO3 and their structure and dielectric property
Ding Shiwen,Wang Jing,Chai Jia, Ding yu
(College of Chemistry and Environmental Science, HeBei University, Baoding 071002)
Received Sept. 15, 2003; Supported by the Natural Science Foundation of Hebei province (No. 299078).
Abstract A series of Ba1-xSrxTiO3 ((0¡Üx¡Ü0.1) solid solutions were synthesized by Microwave method. XRD and cell parameters-component figures of the series of the solid solutions powder demonstrated that the compounds are mutually miscible in the solid solutions. Furthermore, an observation through a TEM shows the products to have a shape of uniform, substantially spherical particles with an average particle size of 40 nm in diameter. The results of preparing ceramics showed that after adulterating with Sr2+ in pure BaTiO3 phase, the dielectric constant increased three times and dielectric loss 2 times lower than those of pure BaTiO3 phase at room temperature. The sintering temperature of nano-powder was decreased 200-250ºC compared to that of micro-powder.Keywords microwave synthesis£¬BaTiO3chemi-adulteration£¬Nano-material , dielectric property Currently the electric components are more and more in light-duty, thin-type and microminiature. Increasing the dielectric constant at room temperature, decreasing dielectric loss and temperature coefficient are very important, and coming to be researchful hotspot in scientific domain. BaTiO3 is a strong dielectric material, which is widely used in the production of electric components such as ceramic capacitor, PTC, medium amplifier, and is indispensable in electronics industry[1]. It has the highest dielectric constant at 120 ºC (~ 104), while its dielectric constant at room temperature is only 1/6 of the Curie point, which greatly limited its practical applications. It is hypothesized theoretically that the Curie point of BaTiO3 may be lowered and broadened when the large Ba2+ ions are partially replaced by small-sized Sr2+[2]. At present, solid phase adulteration was adopted in the production process of electric component in industry. The oxides of Sr, Zr and some rare earth elements are adulterated in BaTiO3 to improve its property, but the solid phase adulteration is not homogeneous and the expected improvements of the parameters for the component can not be achieved. Although a Sol-Gel method for adulterating BaTiO3 has been reported in recent years, its wide use is limited by the high price of the materials[3].Now, many researchers exercise microwave technique in a lot of chemical fields, and have obtained fruitful success. In 1988, microwave method is first used to synthesize inorganic compounds and superconductor materials by Baghurst of Oxford[4]. In 1992, microwave method to synthesize ABO3-type compounds is reported by Komarneni[5]. In succession, synthesizing zeolite molecular screen[6,7], inorganic materials[8,9] and so on are also reported. As a kind of energy source, microwave is stepping in chemical industry, new materials and other scientific and technological fields with incredible speeds. In this paper, BaTiO3 is adulterated to improve its performance by microwave reaction. In this work, the adulterated ions can evenly enter into the matrix lattice. A series of BaTiO3-based solid solutions are synthesized, and their structure and dielectric properties are studied.
1 EXPERIMENTAL
1.1 Material and apparatus
TiCl4£¬Ba(OH)2·8H2O£¬Sr(OH)2·8H2O are of analytical grade and used. All experiments were carried out in double-distilled water.
Rigaku D/MAX-RC-X-ray diffractometer(Jp), TEM-1000SX Transmission Electrical Microscope (Jp),769YP-242 Table Oil Press(Tianjin), RJXG-5-13 Electrical Stove, Automatic LCR Meter(En), Whirlpool microwave stove(Am) and reactor etc.769YP-242 Table Oil Press, RJXG-5-13 Electrical Stove, Automatic LCR Meter and reactor etc.
1.2 Syntheses of Ba1-xSrxTiO3 solid solutions
Hydrolysis of TiCl4 is similar to the literature [10,11]. H2TiO3 is launched into water, and conducted into serum of 250ml,then commixed with 200ml alkaline solution of proportioned Ba(OH)2 and Sr(OH)2. Then the mixture placed in a beaker (The beaker is full of water) is lain in the microwave stove. The heating power is turned to 10 by regulating the heating-power knob for 5 min, then turned to 2 for 15 min, so that the reactant maintains slightly boiled at 100ºC. The solid solutions powder of Ba1-xSrxTiO3 (the values of x are 0.01, 0.02, 0.04, ... ,0.1, 0.2,...,0.9,1 respectively) were obtained after filtering, washing and drying at 100ºC.
1.3 Preparation of ceramics
The ceramic was prepared by the solid solution powder synthesized and their properties were studied. The sample was mixed with suitable amount of adherent (8% PVA aqueous solution) and grounded evenly, then bolted by the screen with its aperture of 0.45 mm (40 mesh). The resulting powder was pressed into disc whose diameter is 1.5 cm and thickness is 0.2cm at a pressure of 6 - 8 Mpa. The binder was removed by firing at 600ºC in air. After cooling, the discs were sintered by heating to 1150ºC. After layed on Ag electrodes, their electric capacity value (C) and dielectric loss ( tand ) were measured by Automatic LCR Meter at different temperatures, their thickness and diameter are measured with micrometer, and their dielectric constants were calculated from e =C· h/e o· S, where C is capacity value of sample, h the thickness of disc, S the electrode area, eo the vacuum dielectric constants, eo=1/4p¡Á 9¡Á1011F/cm.
2 RESULTS AND DISCUSSION
2.1. Effects of reaction time on granularity of Ba1-xSrxTiO3
The dimension of Bao.92Sr0.08TiO3 diminishes with reaction time being prolonged. When reaction time is 20min,the minimum dimension of particles is obtained, which is 40nm. But when reaction time continues to protract, the dimension of particles will largen again. Table 1 Effect of reaction time on granularity of Ba1-xSrxTiO3
Reaction time/min |
5 |
10 |
15 |
20 |
25 |
30 |
Granularity |
70 |
56 |
47 |
40 |
44 |
53 |
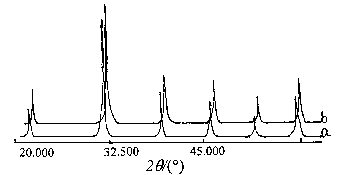
2.2 XRD analyses of the Ba1-xSrxTiO3 solid
solutions
Ba1-xSrxTiO3 solid solutions powder is analyzed with
XRD. It has the same XRD pattern as pure BaTiO3 phase and both of them belong
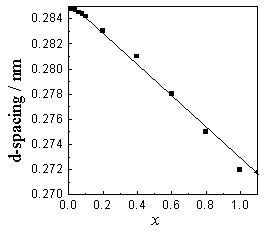
to the cubic system. In Figure 1,with the increasing of x, the d-spacing decreases,
consistent withthe fact that the radius of Sr2+ ion (0.113 nm) is smaller than
that of Ba2+ ion (0.135 nm). To further demonstrate the property of the solid
solution, d-spacing (101 plane) of different disc was measured, and an approximate linear
line is obtained ( Figure 2), which is consistent with the Vegards's law, i.e. unit cell
parameters change linearly with the composition[2]. This proved that the solid
solutions are mutually miscible.
An observation of the Ba1-xSrxTiO3 solid solutions powder by TEM revealed that the solid solution particles are substantially spherical with the average size of 40 nm in diameter, which is shown in Figure 3.
2.4. The relationship between the properties and component of Ba1-xSrxTiO3
The dependences of dielectric properties,e and tand , on the composition of Ba1-xSrxTiO3(0¡Üx¡Ü0.5) solid solution sintered at 1150ºC are shown in Figures 4. It can be seen from the figure that after the adulterated ions Sr2+ evenly enter into the matrix lattice of BaTiO3, which results in Curie point (Tc) of BaTiO3 be lowered, and dielectric constant enhanced from 2000 to 7000, approximately 3 times than that of pure BaTiO3 phase, and dielectric loss at room temperature reduced to 0.01, approximately a half of that of pure BaTiO3 phase. This is because the replacement of Ba2+ (0.135 nm) with Sr2+ (0.113 nm) caused the unit cell parameters to become smaller, which limited the freedom of Ti4+, thus makes the ceramic material exhibit higher dielectric constant and lower dielectric loss at room temperature[10,11].
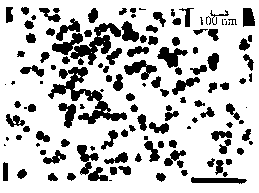

Figure 3 TEM Photograph of Ba0.8Sr0.2TiO3 Figure 4 Relation between a-e, b- tant d and the component of Ba1-xSrxTiO3 2.5 Effects of sintering temperature and time on dielectric properties
The effects of sintering temperature and time on dielectric properties of Bao.92Sr0.08TiO3 can be seen in Table 2 and 3.
The dielectric constant of the sample increases evidently with the augment of temperature until the largest value reached at 1150 ºC, and reduced gradually as shown in Table 2. This is because the ceramic disc is an aggregation of minute grains. In the course of sintering, with the temperature rising, the interface of grains is aggrandized and pores are gradually removed, which results in ceramic disc compacted. The density of disc is the largest, while sintered at 1150ºC, and dielectric constant of the material is the largest and dielectric loss is the smallest at room temperature. When temperature exceeds 1150 ºC, recrystallizing can be observed, which makes large crystal formed and relatively larger closed pore structure in crystals. This results in dielectric constant reduced and dielectric loss increased. Sintering time has the same effects on dielectric properties of the materials shown in Table 3.
Table 2 Effect of sintering temperature on dielectric property
t/ ºC |
1050 |
1100 |
1150 |
1200 |
1250 |
1300 |
¦Å |
4380 |
6300 |
7200 |
6610 |
5570 |
5120 |
¦Ä |
0.39 |
0.026 |
0.01 |
0.012 |
0.036 |
0.081 |
t/h |
0.5 |
1 |
2 |
2.5 |
3 |
3.5 |
¦Å |
5620 |
6870 |
7200 |
6730 |
5820 |
4760 |
¦Ä |
0.023 |
0.018 |
0.01 |
0.016 |
0.046 |
0.067 |
A series of Ba1-xSrxTiO3 solid solutions were synthesized in aqueous solution by microwave method. The synthesis temperature is lowered by 1150 ºC and 750ºC compared with the traditional solid phase method (1250ºC), oxalate method and Sol-Gel method (Sintering temperature is 850ºC). Reaction time is shortened from 5h of hydrothermal method to 20 min.
After adulteration of appropriate amount of Sr2+ in BaTiO3 by chemical method, the adulterated ions entered into the matrix lattice evenly. The material has a higher dielectric constant, 3 times larger than that of pure BaTiO3 phase, and its dielectric loss is one half lower than that of BaTiO3 at room temperature.
A process of nano-powder sintering is probed. The sintering temperature decreases 200-250 ºC compared to traditional large powder (micron)[12], which makes low-temperature sintering of MLC and other electric apparatus into reality. REFERENCES
[1] Su W. Inorganic Chemicals Industry ( Wujiyan Gongye ), 1992, (3): 5.
[2] West A R. Solid State Chemistry And Its AppliSrtions. New Delhi: John Wiley Sons Ltd., 1984: 535.
[3] Heistand R H, Duquette L G, Skeele F P. Ceram. Trans, 1988, (2) : 94.
[4] Baghurst D R, Chippindale A M, Mingos D M P. Nature, 1988, 332 : 311.
[5] Komarneni S, Roy R, Li Q H. Mat. Res. Bull£¬1992, 27 : 1393.
[6] Du H B, Fang M, Xu W G. J. Mater. Chem, 1997, 7(3): 551.
[7] Song T Y, Xu J N, Xu W G. Chemical Journal of Chinese Universities ( Gaodeng Xuexiao Huaxue Xuebao ), 1992, 13: 1209.
[8] Pang G S, Cui D L, Xu R R. Acta Chim. Sinica (Huaxue Xuebao), 1996£¬54 : 575.
[9] Feng S H, Cui D L, Xu R R. Chemical Journal of Chinese Universities ( Gaodeng Xuexiao Huaxue Xuebao ), 1996, 17: 1495.
[10] Ding S W, Zhai Y Q, Li Y. Sci. China Ser. B, 2000£¬43:283.
[11] Ding S W, Zhai Y Q, Li X M. Acta Chim. Sinica (Huaxue Xuebao), 2000, 58: 1139.
[12] Xu T X, Bo Z M, Fang C X. Electric Ceramic Materials ( Dianzi Taoci Cailiao ). Tianjin: Tianjin University press,1993 : 161.
¡¡
¡¡