http://www.chemistrymag.org/cji/2003/05c089pe.htm |
Dec. 1, 2003 Vol.5 No.12 P.89 Copyright |
Coordination of Nitrogen containing polymer with Cu (II) ion and its Catalysis to Methyl Methacrylate
Guo Yeshu1, Lu Jianmei*1 2, Wu Jianfei1, Xia Xuewei1, Zhu Xiulin1(1Department of Chemistry and Chemical Engineering, Suzhou University, Suzhou China 215006; 2Jiangsu Polytechnic University, Changzhou, China, 213016)
Received Sep. 22, 2003; Supported National Natural Science Foundation of China (No. 20076031) and Natrural Science Foundation of Jiangsu Province (No. BK2002042)
Abstract The
methacrylic 2-(dimethylamino) ethyl ester(DM)was polymerized under microwave irradiation,
and then PDM was directly coordinated with Cu2+ to form Polymer-Metal Complexes
PDM-Cu. IR, ESR, XPS, TGA, DSC were employed to analyze the structure of PDM-Cu and its
catalytic function to Methyl Methacrylate (MMA) polymerization was studied. GPC was used
to characterize the obtained polymer. The kinetics of MMA polymerization catalyzed by
Cu-PDM was studied. The result showed that the maximum inherent viscosity of PDM
polymerized under microwave irradiation was 37.75ml/g, and the maximal conversion
was 92%. The PDM-Cu2+complex can heterogeneously catalyze the polymerization of
MMA at room temperature and the maximum molecular weight of obtained PMMA was 830,000.
Keywords Microwave irradiation, polymerization, Methacrylic
2-(dimethylamino) ethyl ester, catalyze, MMA
In this paper microwave irradiation was applied to synthesize PMC such as Cu-PDM,and the catalytic function of Cu-PDM was studied and the kinetics of MMA polymerization catalyzed by Cu-PDM was studied. Herein methacrylic 2-(dimethylamino) ethyl ester (DM) was used to synthesize PMC. During the polymerization, the C=C was opened and formed the main chain, while N was located on side chain, so the obtained PMC was side-chain metal complex. 1 EXPERIMENT SECTION
1.1 Base Material
Methacrylic 2-(dimethylamino) ethyl ester(DM), Methyl Methacrylate(MMA), methyl alcohol, N,N-dimethyl formamide (DMF), Chemical grade, cyclohexane, chloroform, tetrahydrofuran, sodium sulfite, blue vitriod and hydrochloric acid: analytical grade.
1.2 Device
Xianhua microwave, KS2163, the maximum output power is 850 W, refitted. The microwave oven electric current could be adjusted and read continuously by using voltage regulator, which installed outside of the oven. The reaction device is showed as Figure 1.

Figure1 Refitted microwave device
1.3 Procedure of experiment
1.3.1 Polymerization of DM
(1) Polymerization of DM under microwave irradiation
[Note1] DM homo-polymer was poured into cyclohexane 20 times of the quantity of polymerization system. PDM was precipitated and filtered through the cyclohexane.
(2) Traditional heating polymerization of DM
Weighed DM was added into three-necked flask, pick up the suction and filled with N2 for 30 minutes, and then put into the hot bath which has been heated to 85¡À1ºC, started the heating polymerization with protection by N2 all the time, the temperature was still controlled at 85¡À1ºC,After set time, the mixture was took out and treated with specified solvents as process of Note 1. Finally, it was washed out, dried up until to constant weight under vacuum at low temperature.
1.3.2 Synthesis of Cu-PDM
CuSO4 aqueous solution was added into the PDM prepared as part3 (1) in this paper, and the mixture was set for 24 hours to improve the coordination degree between the PDM and metallic ion, then it was separated through centrifugal and uncoordinated Cu2+ was removed by deionized water through repeated wash with deionized water. The product above mentioned was dried up until to constant weight under vacuum at low temperature.
1.3.3 Copper content analysis through atomic absorption spectroscopy
PDM-Cu(II) complex was washed by diluted hydrochloric acid(1:1), then filtered. The copper content in the filtrate was determined through atomic absorption spectroscopy. The result showed copper content in PDM-Cu(II) was 3.04%.
1.3.4 MMA polymerization in the presence of Cu-PDM
A certain weight of MMA was added into a conical flask, and then Cu-PDM/Na2SO3 aqueous solution was added in certain ratio. Methyl alcohol containing a certain concentration of hydrochloric acid was added into the flasks to precipitate the PMMA, which was then repeatedly filtered, washed with deionized water and dissolved in THF. A certain hydrochloric acid was added in order to separate the PMC, dissolving Cu2+, which was in PMC into water. PMMA was dried up to constant weight, and PMMA's viscosity and molecular weight was determined by using GPC.
1.3.5 Investigation of kinetics MMA polymerization catalyzed by Cu-PDM /Na2SO3 aqueous solution.
MMA was poured into 100ml round bottom flask placed in a bath at 45ºC, and 60 ml aqueous solution of 0.2g Cu-PDM and 0.2g Na2SO3 was added as catalyst. A dilatometer was installed onto the top of the flask.
1.3.6 Measurement
a. IR spectrum: determined by American Perkin ¨C Elmer 577 (KBr pressed disc method))
b. ESR: ER2000-SRC (Bruker)
c. XPS:V.G.. Scientific ESCALAB Mk(II) (U.K.)
d. GPC: Waters 150c GPC.
e. Thermal analysis system: DELTA SERIES TGA7, American P-E. Temperature range: room temperature¡ª950ºC; Sensitivity: 0. 1mg, heating Rate: 10. 0ºC/min.
f. Atomic absorption spectrometer: Hitachi 180-80 Polarized Zeeman Atomic absorption spectrophotometer. 2 RESULT AND DISCUSSION
2.1 The polymerization of DM under microwave irradiation
The reaction scheme was showed below:

2.1.1 Influence of microwave
irradiation power on the inherent viscosity of polymer
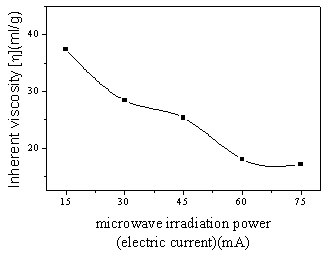
As Figure 2 showed the inherent viscosity of PDM was found to deal with
increasing microwave irradiation power. In the range of experiment, when the microwave
irradiation power was controlled by electric current at 15mA, the inherent viscosity of
polymer reached maximum. The inherent viscosity decrease was due to the increase of
radical number result from increase of microwave irradiation energy.
2.1.2 Productive rate of PDM
under microwave irradiation:
(1) Influence of microwave irradiation time to the yield of PDM:
As Figure 3 showed, at a certain microwave irradiation power, with electric current
controlled at 15mA, the PDM yield increased with the increasing of irradiation time. In
the range of this experiment, results show that the yield of polymerization can reach the
maximum value 92%,when the polymerization time is belonged to 2.5 hours.
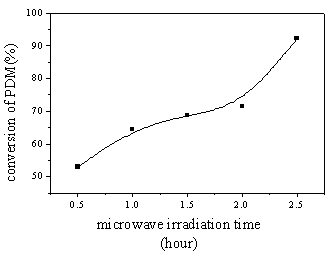
Figure3 Relationship between microwave
irradiation time and conversion of PDM
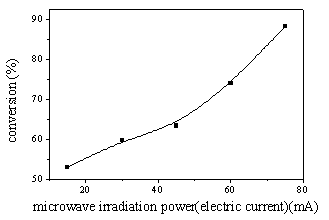
Figure4 Relationship
between microwave irradiation power and conversion of DM
(2) Influence of microwave
irradiation power to the productive rate of PDM
As Figure 4 showed, the yield of polymerization system increased with the increasing of
microwave irradiation power at the same reaction time. In the range of this experiment,
when the microwave irradiation power was controlled at 75 mA, the yield of PDM reached the
maximum value of about 88%.
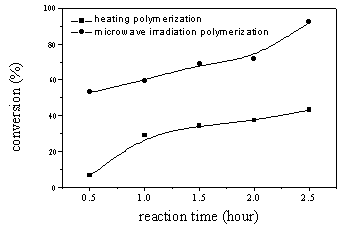
(3) Influence of traditional heating polymerization time of DM to the productive rate of
PDM
As Figure 5 showed, during the traditional heating polymerization, when reaction time was
0.5 hour and the reaction temperature was 85¡À10C, conversion of DM was 6%. In
the range of this experiment, when the polymerization time was 2.5hour, the conversion of
DM reached the maximum value 43%. The comparison of two type heating method of synthesis
of PDM in the Fig5, it showed that the yield obtained by microwave irradiation method was
far greater than the one obtained by traditional heating method.

Figure 6 IR spectrum of PDM

Figure 7 IR
spectrum of Cu-PDM
2.2.1 IR spectrum
Figure 6 was the IR spectrum of PDM, and Figure7 was the IR spectrum of Cu-PDM complex. From the Figure7, it showed that a strong peak appeared at 1130.4 cm-1, it should be migration of VC-N peak 1161.2cm-1 resulted from the coordination of N of the co-polymer with Cu2+. The change of the IR peak was confirmed that coordination of Cu(II) with N which is in the co-polymer PDM and to form Cu-PDM[17].
2.2.2 ESR spectrum of Cu-PDM
As Figure 8 showed, on the ESR curve of the Cu-PDM, three g value appeared. The first g=2.907, the second g=2.183 and the third g=2.036. The polymer itself has no ESR sign, and Cu2+ has a g=2.190[18]. Since the change of ESR sign position or change of g value is due to the nature of chemical bond[19], it could be inferred a new coordinate bond formed between Cu2+ and polymer.
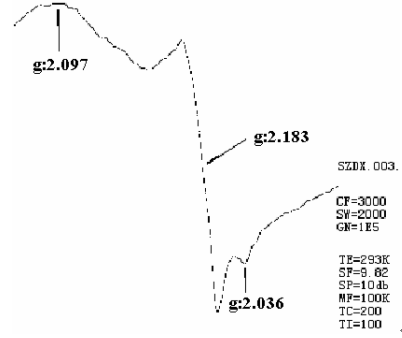
Figure 8 ESR spectrum of Cu-PDM
2.2.3 XPS Spectra
The XPS binding energies were obtained from the Cl(2p), N(1s), Cu(2p) core levels
of PDM-Cu(II) and CuCl2·2H2O are listed in Table1. The
binding energy of Cu2P1/2, Cu2P2/3 in CuCl2·2H2O is 954.6eV and 934.2eV.
While the binding energy of Cu2P1/2, Cu2P2/3 in PDM-Cu (II) are 952.6 eV and 932.8eV
respectively. After coordination the 2p electron's binding energy of Cu (II) Cu2p3/2,
Cu2p1/2 were lower than the electron's binding energy before coordination by 1.4eV and
2.0eV, respectively. While the binding energy of N (1s) in PDM-Cu (II) was higher than
that in PDM by 1.0 eV. This is due to the electronic cloud shift from N to Cu. When
electronic cloud shift from one to other atoms, the former¡¯s nuclear charge will increase, contrarily the nuclear charge will
decrease.
Table 1 Electron Binding energy (eV) of XPS of PDM and PDM-Cu(II)
Sample |
Cl(2p) |
N(1s) |
Cu (2p) |
|
| Master peak | ||||
| 2p3/2 | 2p1/2 | |||
PDM |
399.6 |
|||
| CuCl2 2H2O | 199.2 |
934.2 |
954.6 |
|
PDM-Cu (II) |
198.2 |
397.8 |
932.8 |
952.6 |
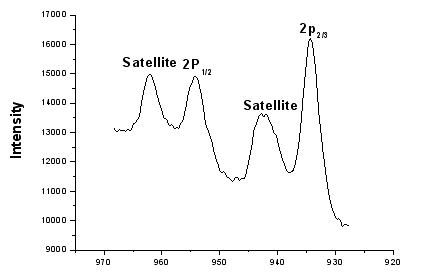
Figure 9 Binding Energy (eV) of Cu2P in CuCl22H2O
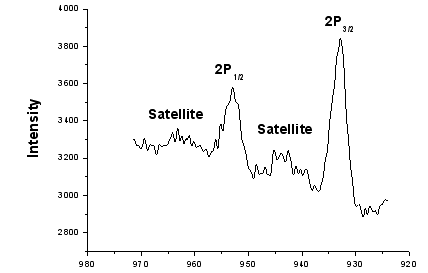
Figure 10 Binding Energy (eV) of Cu2P in PDM-Cu(II)
2.2.4 Thermal analysis
(1) Thermogravimetric analysis (TGA)
From the Figure11, the thermogravimetric curve showed that,from the start temperature to
270
(2) Differential scanning calorimetry (DSC)
From the Figure 12, there was an obvious exothermic peak of Cu-PDM at 285ºC, it was in accordance with its TGA curve, a compose of the complex occurred at this temperature, which resulted from the breaking of coordination bond.
From the before analysis, it showed that the PDM coordinated with the Cu 2+.
 Temperature (ºC) DELTA SERIES TGA |
 Temperature (ºC) DELTA SERIES DSC |
| Figure 11 TGA of Cu-PDM | Figure12 DSC of Cu-PDM |
The polymerization of MMA was examined at room temperature in the presence of water. It was found that the MMA could not polymerize when only Na2SO3 or 5ml Cu-PDM aqueous solution was added alone into 10g MMA, while Na2SO3 and Cu-PDM existed at the same time MMA started to polymerize after about 3 minutes at room temperature.
(1) Polymerization kinetics of MMA initiated by Cu-PDM/Na2SO3 system
The polymerization mechanism of MMA initiated by Cu-PDM/Na2SO3 system Yang[22]etal found that the copper polycarboxylate/sodium sulfite can catalyze polymerization of MMA in a complexation way. Cu-PDM/Na2SO3 system catalyzed MMA polymerization in a similar way, since the color of Cu-PDM/Na2SO3 did not change, from which we could exclude the oxidation-reduction mechanism. The reaction mechanism can be showed as Figure13.
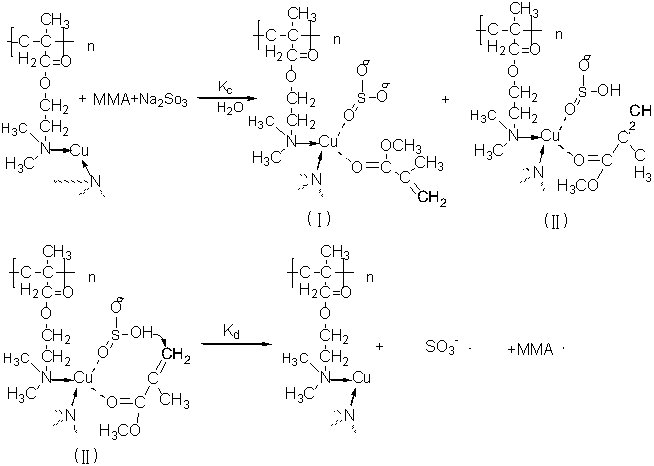
Figure 13 The polymerization mechanism of MMA initiated by Cu-PDM/Na2SO3 system
the kinetics of polymerization could be
formulated as follows [23].
![]()
At a lower monomer conversion, the concentration of free radicals was constant, so the
formulation was described as:
[M0] is the initial monomer concentration, [M] is monomer concentration at time t. K is a constant when temperature keep constant.
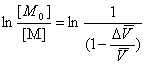
In all, the formulation could be deduced as follows,
Based on the kinetics determination of MMA polymerization initiated by Cu-PDM /Na2SO3 aqueous solution, it displayed that the polymerization was in accordance with free radical mechanism , Rp=68.5*10-5mol/ls.
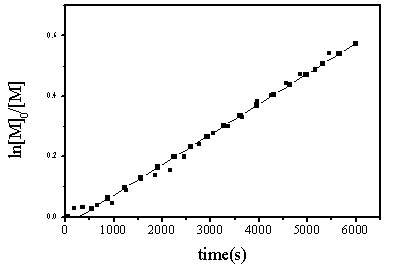
Figure 14 Kinetics determination of MMA catalyzed by Cu-PDM/Na2SO3 aqueous solution system
(2) Influence of the content of Cu-PDM and Na2SO3 to the polymerization of MMA
The influence of Na2SO3 content on

Figure 15 Relationship between content of Na2SO3 and Mw of PMMA 4 CONCLUSION
The homo-polymerization of DM can be prompted by the microwave irradiation, which shorten the reaction time and improved the yield of PDM. Microwave irradiation power decreased the inherent viscosity of PDM resulted from the increase of free radicals number. The PDM obtained by microwave irradiation can coordinate with the Cu2+ directly to form Cu-PDM. The Cu-PDM could heterogeneously catalyze MMA polymerization at room temperature in a free radical mechanism. The Cu-PDM/Na2SO3 system has a high catalytic activity when catalyzing MMA polymerization. The molecular weight of PMMA obtained by catalysis of Cu-PDM can reach 830,000, which was greater than that obtained by traditional initiator. The solid catalyst could be easily separated from the polymerization system. REFERENCES
[1] Gedye R N, Smith F E, Westaway K A et al. Tetrahedron Lett., 1986, 27: 279.
[2] Giguere R J, Bray T L, Duncan S M et al. Tetrahedron Lett., 1986, 27: 4945.
[3] Teffal M, Gourdenne A. Eur. Polym. J., 1983, 19: 543.
[4] Stoffer J O, Sitatram S P. Am. Chem. Soc., Proc. Polym. Mater. Sci. Eng., 1994, 71: 55.
[5] Dori A D, Huggett R, Bates J F et al. Dent. Mater., 1988, 4: 25.
[6] Mijovic J, Wijaya J. Polym. Compos., 1990, 11: 84.
[7] Thuillier F M, Jullien H, Grenier-Loustalot et al. Polym. Commun., 1986, 27: 206.
[8] Lewis D A, Hedrick J C, McGrath J E et al. Am. Chem. Soc., Polym. Prepr., 1987, 28 (2): 330 .
[9] Silinski B C, Kuzmyca, Gourdenne A. Eur. Polym. J., 1987, 2: 273.
[10] Jullien H, Valot H. Polymer, 1985, 26: 506.
[11] Silinski B C, Kuzmycz A, Grourdene. Eur. Polym. J., 1987, 2: 273.
[12] Kishanprasad V S, Gedam P H. J. Appl. Polym. Sci., 1993, 50: 419.
[13] Lu J M, Zhu X L. J. Appl. Polym. Sci., 1998, 68: 1563.
[14] Lu J M, Zhu X L. J. Appl. Polym. Sci., 1997, 66: 129.
[15] Baghurst D R, Cooper S R, Greene D L et al. Polyhedron, 1990, 9: 893.
[16] Michael D, Mingos P. J. Chem. Soc. Chem. Commun., 1996, 899.
[17] Li Q L, Chi X Z, Zeng Y H. Instrument analysis. Beijing Normal School publishing company, Beijing, 1990, 148.
[18] Robinson, J W, Handbook of Spectroscopy VII, 253.
[19] You X Z. Instruction of structure analysis. Beijing: Science Publishing Company, 1980, 520.
[20] Xiansu Cheng, Huaimin Guan, Yingcao Su. Journal of Inorganic and Organometallic Polymers, 2000, 10 (3): 115-126.
[21] Collins E A, Bares J, Billmeyer F W. Experiment of Polymer Science, Beijing: Science Publishing Company, 1983, 264.
[22] Yang C X, Lin L, Wu J Y. Macromolecule Transaction, 1989, 1: 12.
[23] Polymer teaching and research group of chemitry department in Fudan University, Experimental technique of macromolecule, Fudan University Publishing Company, 1983, 165.
¡¡