http://www.chemistrymag.org/cji/2003/05c090pe.htm |
Dec. 1, 2003 Vol.5 No.12 P.90 Copyright |
(Guangxi Traditional Chinese Medical University, Nanning 530001, China)
Received Jul. 29, 2003; Supported by the Science Foundation of Guangxi Traditional Chinese Medical University (No. G200217) and the Science Foundation of Guangxi (No. 0342003-5) .
Abstract Three novel mononuclear ruthenium(II) complexes [Ru(L)2NOP]2+ [L= 1,4,8,9-tetra-aza-triphenylene(tatp), dipyrido[3,2-a:2',3'-c]phenazine(dppz) and benzo[i]dipyrido [3,2-a:2',3'-c]phenazine(dppn), NOP = 2-(4-nitrophenyl)-phenylimidazo[4,5-f][1,10]phenanthro- line] have been synthesized and characterized with microanalysis, ES-MS, NMR, and UV-Vis. Two routes were exploited to synthesize [Ru(L)2(NOP)]2+ (L=dppz, dppn). The electrochemical behaviors of these complexes in acetonitrile have been studied by cyclic voltammetry. The nonlinear optical properties of the ruthenium(II) complexes were investigated by Z-scan techniques with 12 ns laser pulses at 540 nm, and all of them exhibit both NLO absorption and self-defocusing effect. The corresponding effective NLO susceptibility | c3
| of the complexes are 8.65¡Á10-12, 9.75¡Á10-12, 10.8¡Á10-12 esu. The results indicate that the extension of the p framework of the ligands can enhance NLO properties of the complexes.Keywords Ruthenium(II) complexes, Polypyridine ligands, NLO properties
1 INTRODUCTION
The octahedral polypyridyl complexes have received considerable attention[1]
since Paris and Brandt[2] discovered [Ru(bpy)3]2+
visible-region luminescence at 77K in 1959. The NLO materials studies of ruthenium
complexes are also the subject of considerable investigations[3] since the
second-order nonlinear susceptibity of [Ru(bpy)3]2+ and [Ru(phen)3]2+
was first demonstrated by Zyss et al[4]. However the studies have mainly
concentrated on the quadratic nonlinear properties[5], the third-order NLO
properties of this kind complexes are received little attention[6]. The
phenanthroimidazolebenzene derivatives are candidates for the third-order NLO materials in
virtue of their high third harmonic generation and rapid response[7]. The
structure-property relationships that govern third-order NLO polarization are a
little vague[8], however, for the manifestation of large third-order
nonlinearities, p-electron
delocalization must be present in the molecule[9]. The
2-(4-nitrophenyl)phenylimidazo-[4,5-f][1,10]phenanthroline(NOP) is analogous to
phenanthroi- midazolebenzene and has a larger
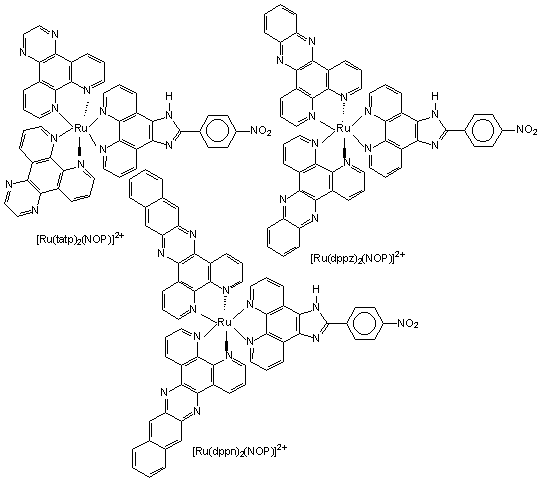
Figure 1 Structures of the three new complexes
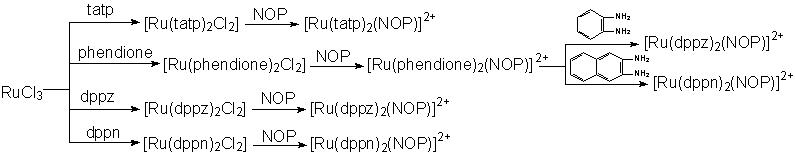
Scheme 1 The two synthetic route of the new complexes
2 EXPERIMENTAL
2.1 Materials and preparations
The compounds 1,10-phenanthroline-5,6-dione[11], NOP[11],
1,4,8,9-tetra-aza-triphenylene (tatp)[11], dipyrido[3,2-a:2',3'-c]phenazine(dppz)[12]
and benzo[i]diprido[3,2-a:2',3'- c]phenazine(dppn)[12] were
synthesized according to the literature methods. Other materials were commercially
available and of reagent grade.
(Caution: perchlorate complexes are potential explosives. These complexes must be
handled in small quantity and with great care.)
2.1.1 Synthesis of cis-[Ru(tatp)2Cl2] H2O. It was
synthesized in a similar manner to that described in the literature methods[13].
A mixture of RuCl3 3H2O (1 mmol, 0.26 g), tatp(2 mmol, 0.464 g),
LiCl (6.7 mmol, 0.4 g) and DMF (6 cm3) was refluxed under argon for 8 h during
which the solution color changed from purple to purple black. Yield 70.3%. (Found: C,
50.2; H, 3.1; N, 16.8%. Calc. For C28H20N8 O2Cl2Ru:
C, 50.0; H, 3.0; N, 16.7%).
2.1.2 Synthesis of cis-[Ru(dppz)2Cl2]
2.1.3 Synthesis of cis-[Ru(dppn)2Cl2]H2O. It was synthesized in a similar manner to that described for cis-[Ru(tatp)2Cl2] H2O, with dppn (2 mmol, 0. 664 g) in place of tatp. Yield 71.5%. (Found: C, 61.6; H, 3.1; N, 13.2%. Calc. For C44H26N8OCl2Ru: C, 61.8; H, 3.1; N, 13.1%).
2.1.4 Synthesis of cis-[Ru(phendione)2Cl2]2H2O. It was synthesized in a similar manner to that described for cis-[Ru(tatp)2Cl2] H2O, with 1,10-phenanthroline-5,6-dione (2 mmol, 0. 42 g) in place of tatp. Yield 69.2%. (Found: C, 45.6; H, 2.8; N, 8.4%. Calc. For C24H16N4O6Cl2Ru: C, 45.9; H, 2.6; N, 8.5%).
2.1.5 Synthesis of [Ru(tatp)2(NOP)](ClO4)2H2O (1). A mixture of cis-[Ru(tatp)2Cl2]2H2O (0.2 mmol, 0.123 g), NOP(0.2 mmol, 0.064 g) and ethylene glycol (30 cm3) was refluxed under argon for 5h during which the solution colour changed from purple to deep red. The solution was cooled to r.t., and 60 cm3 H2O was added. After filtration, a dark red precipitate was obtained by dropwise addition of aqueous NaClO4 solution to the filtrate. The product was purified by column chromatography on alumina using acetonitrile as eluent then dried in vacuo. Yield 69.2%. (Found: C, 50.1; H, 2.4; N, 16.2%; Calc. For C47H29N13O11Cl2Ru: C, 50.2; H, 2.6; N, 16.2%). ESMS [CH3OH, m/z]: 908 ([M-2ClO4-H]+), 404.5 ([M-2ClO4]2+). 1H NMR[500 MHz, (CD3)2SO]:d9.52(m, 4H), 9.36(s, 4H), 8.97(d, 2H), 8.56(d, 2H), 8.35(d, 1H), 8.34(d, 1H), 8.32(d, 2H), 8.23(d, 1H), 8.22(d, 1H), 7.91(m, 6H), 7.65(t, 2H).
2.1.6 Synthesis of [Ru(phendione)2(NOP)](ClO4)2 0.5H2O. A mixture of cis-[Ru(phen dione)2Cl2] 2H2O (1 mmol, 0.628 g), NOP (1 mmol, 0.341 g).and ethylene glycol (30 cm3) was refluxed under argon for 6h. The solution was cooled to r.t., and 60 cm3 H2O was added. After filtration, a brown precipitate was obtained by dropwise addition of aqueous NaClO4 solution. The product was purified by recrystalization from MeCN-Et2O and then dried in vacuo. Yield 58.3%(Found: C, 50.5; H, 2.5; N, 12.2%; Calc. For C43H24N9O10.5Cl2Ru: C, 50.8; H, 2.4; N, 12.4%).
2.1.7 Synthesis of [Ru(dppz)2( NOP)](ClO4)2 H2O (2). (A) the direct synthesis method. This complex (deep red) was synthesized in a similar manner to that described for [Ru(tatp)2(NOPH)](ClO4)2 H2O, with cis-[Ru(dppz)2Cl2] H2O (0.2 mmol, 0.14 g) in place of cis-[Ru(tatp)2Cl2] 2H2O. Yield 28.2%. (Found: C, 53.8; H, 2.5; N, 14.66%; Calc. For C55H33N13O11Cl2Ru: C, 54.0; H, 2.7; N, 14.9%). (B) the condensation synthesis Method[14]. The complex is also synthesized by the following method to attain a higher yield. A mixture of [Ru(phen-dione)2(NOP)] (ClO4)2 0.5H2O (0.1 mmol, 0.102 g), O-phenylenediamine(0.4 mmol, 0.043 g), EtOH(50 cm3) and MeCN(50 cm3) was refluxed under argon for 8h. The solution was concentrated to 20 cm3. After filtration, a deep red precipitate was obtained by addition of 50 cm3 Et2O to the filtrate. The product was purified by recrystalization from MeCN-Et2O then dried in vacuo. Yield 44.6%.(Found: C, 54.2; H, 2.4; N, 14.6%; Calc. For C55H33N13O11Cl2Ru: C, 54.0; H, 2.7; N, 14.9%). ESMS [CH3OH, m/z]: 1006 ([M-2ClO4-H]+), 503.5 ([M-2ClO4]2+). 1H NMR[500 MHz, (CD3)2SO]:d9.62(dd, 2H), 9.19(s, 2H), 9.08(s, 2H), 8.57(s, 1H), 8.55(s, 1H), 8.54(d, 2H), 8.47(d, 4H), 8.38(t, 2H), 8.29(dd, 2H), 8.18(t, 4H), 8.06(t, 2H), 7.92(m, 4H), 7.77(m, 2H).
2.1.8 Synthesis of [Ru(dppn)2(NOP)](ClO4)2 H2O (3). (A) the direct synthesis method. This complex (deep red) was synthesized in a similar manner to that described for [Ru(tatp)2(NOP)](ClO4)2 H2O , with cis-[Ru(dppn)2Cl2] H2O (0.2 mmol, 0.16 g) in place of cis-[Ru(tatp)2Cl2] 2H2O. Yield 24.3%. (Found: C, 57.4; H, 3.2; N, 13.5%; Calc. For C63H38N13O11Cl2Ru: C, 57.1; H, 2.9; N, 13.7%). (B) the condensation synthesis method. This complex (deep red) was synthesized in a similar manner to that described for [Ru(tatp)2(NOP)](ClO4)2 H2O, O-naphthalnediamine with (0.4 mmol, 0.063 g) in place of O-phenylenediamine, and reaction time is 5h. Yield 44%.(Found: C, 57.4; H, 3.1; N, 13.5%; Calc. For C63H38N13O11Cl2Ru: C, 57.1; H, 2.9; N, 13.7%). ESMS [CH3OH, m/z]: 1104 ([M-2ClO4-H]+), 552 ([M-2ClO4]2+). 1H NMR[500 MHz, (CD3)2SO]:d9.57(m, 4H), 9.17(d, 2H), 9.11(d, 2H), 9.02(s, 2H), 8.51(d, 4H), 8.39(d, 4H), 8.35(d, 2H), 8.21(d, 2H), 7.96(s, 4H), 7.75(d, 4H), 7.60(t, 2H), 7.52(t, 2H).
2.2 Physical measurements
The microanalyses (C, H and N) were carried out with a Perkin-Elmer 240Q elemental analyser. US/VIS spectra were recorded on a Shimadzu UV-3101PC spectrophotometer. 1H NMR spectra were measured on a Varian-500 NMR spectrometer with (CD3)2SO as solvent at room temperature and all chemical shifts are given relative to TMS. Electrospray mass spectra (ES-MS) were recorded on a LCQ system (Finnigan MAT, USA) using methanol as mobile phase. The spray voltage, tube lens offset, capillary voltage and capillary temperature were set at 4.50 KV, 30.00 V, 23.00 V and 200 oC, respectively, and the quoted m/z values are for the major peaks in the isotope distribution.
Cyclic voltammetric measurements was performed on an EG&G PAR 273 polarographic analyzer and 270 universal programmer. The supporting electrolyte was 0.1 mol·dm-3 tetrabutylammonium perchlorate in acetonitrile freshly twice distilled from phosphorus pentoxide and deaerated by purging with nitrogen for 0.5 h. A standard three-electrode system composed of a platinum microcyclinder working electrode, platinum-wire auxiliary electrode and a saturated calomel reference electrode (SCE) was used.

Figure 2 Schematic illustration of the experimental set-up for Z-scan measurements.
2.3 Non-linear optical measurements
3. RESULTS AND DISCUSSION
3.1 Synthesis and characterization
The preparation of the dichloro complex, Ru(L)2Cl2(L= tatp,
dppz, dppn) was adopted from the literature[13]. The mixed-ligand
complex 1 is prepared by direct reaction of Ru(tatp)2Cl2 with the
NOP in ethylene glycol in relative high yield. The desired ruthenium(II) complex was
isolated as the perchlorates and purified by column chromatography. Complexes 2 and 3 were
synthesized by direct synthesis method in a similar manner to the complex 1. We used the
condensation synthesis method to synthesize the first key intermediate, [Ru(phendione)2(NOP)]
(ClO4)2 0.5H2O, which has a better solubility than the
complex 2 and 3, then condensated with appropriate o-diamino compounds to obtain the
products in relative high yield.
The 1H-NMR spectra of these ruthenium (II) complexes are
unambiguous identification and assessment of purity, the products have also been
characterized further by elemental analysis and electrospray mass spectrometry.
3.2 Electrochemical studies
The electrochemical behaviors of the complexes have been studied in CH3CN by
cyclic voltammetry. Results are showed in Table 1. Each complex exhibits oxidation (one)
and reduction (two or three) waves in the sweep range from -1.90 to +1.70 V. The anodic
and cathodic peak seperations vary from 60 to 75 mV and nearly scan rate independent,
indicating that the processes are reversible one-electron transfers.
Complex |
RuIII/RuII | Ligand reduction |
||
1 |
1.46 |
-1.13 |
-1.43 |
-1.70 |
2 |
1.38 |
-0.83 |
-1.38 |
-1.65 |
3 |
1.45 |
-1.01 |
-1.45 |
-1.80 |
a All complexes were measured in 0.1 M NBu4ClO4-CH3CN, error in potentials was ¡À0.02 V; T = 23¡À1ºC; scan rate = 100 mV.
Table 2 Absorption spectral data of the ruthenium(II) complexesa
Complex |
l max/nm ( e /M-1 cm-1) |
[Ru(tatp)2NOP]2+ (1) |
470 (sh) 439(39100) 329 (33900) 286£¨83800£©257 (4400) |
[Ru(dppz)2NOP]2+ (2 ) |
472 (sh) 440(1600) 371 (42700) 283£¨142900£© |
| [Ru(dppn)2NOP]2+ (3) | 472 (sh) 440 (41600) 411 (4700) 390 (37700) 326 (151100) |
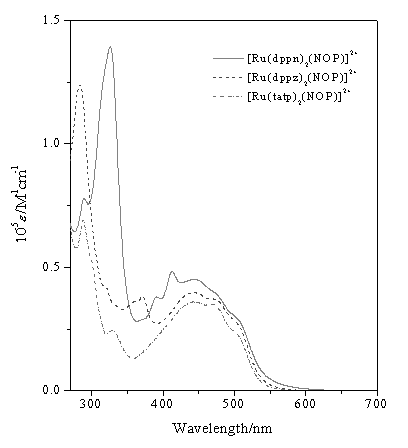
Figure 3 Absorption spectra of the complexes
in DMF at room temperature.
Absorption spectra were measured in DMF for all complexes at room temperature (Fig.3), and the absorption data and the absorption coefficients were showed in Table 2, which are characterized by intense p-p* ligand transitions in the UV and metal-to¨Cligand charge transfer (MLCT) transition in the visible region. The visible spectra typically display two maxima. The MLCT absorption bands maximums(shoulder peak) appear at 470, 472, 472 nm for complex 1, 2, 3 respectively. These absorption spectra can be assigned to Ru(dp) ¡ú NOP (p*) transitions. They are bathochromically shifted by comparison with that of [Ru(phen)3]2+ (447 nm)[15], which can be assigned to the extension of the corresponding p-system. Other intense MLCT absorption bands appear at 439, 440 and 440 nm for complex 1, 2, and 3 respectively. Compared with the [Ru(phen)2DPPZ]2+ (439 nm)[16] , [Ru(phen)2DPPN]2+ (443 nm)[16], these absorption spectra can be assigned to Ru(dp) ¡ú tatp, dppz, dppn (p*) transitions respectively. Their red shift trend in according with the extension of corrsponding p-system is not obvious. Similar cases were observed between [Ru(bpy)2DPPZ]2+ ( 448 nm) and [Ru(DPPZ)3]2+ (455 nm) [17], The authors proposed these to the strong p-accepted phenazine site being only slightly coupled electronically to the ruthenium core and so the complexes show bichromophoric character of [Ru(bpy)3]2+ and phenazine. The cases are believed to resemble these with an extended diimine ligand (tatp, dppz or dppn) site coupled to ruthenium electronically but not strongly.
3.4 Non-linear optical properties
The Z-scan results for the complexes are shown in Table 3. The nonlinear absorption components were evaluated by Z-scan experiment under an open aperture configuration, for example see Fig. 3a. The third-order NLO absorptive process under the conditions can be described theoretically by eqns. (1) and (2) [10].
Table 3 Measurement results of the RuII complexes using Z-scan techniques
Complex |
DTv-p |
I0/W m- 2 |
a /cm- 1 |
n2/m2 W- 1 |
a2/m W- 1 |
c (3)/esu a |
g /esu a |
1 |
0.282 |
2.06¡Á1012 |
2.30 |
-2.10¡Á10-17 |
16.60¡Á10-11 |
8.65¡Á10-12 |
8.65¡Á10-29 |
2 |
0.314 |
2.06¡Á1012 |
2.42 |
-2.37¡Á10-17 |
18.51¡Á10-11 |
9.75¡Á10-12 |
9.75¡Á10-29 |
3 |
0.337 |
2.00¡Á1012 |
2.59 |
-2.60¡Á10-17 |
21.20¡Á10-11 |
10.80¡Á10-12 |
10.80¡Á10-29 |
T(Z) = ![]() (1)
(1)
q(Z) = a 2Ii(Z)![]() (2)
(2)
Where a0 and a2 are linear
and nonlinear absorptive coefficients, L is the thick of the quartz cuvette for
optical measurements and t is the time. Light transmittance (T) is a
function of the sample's Z-position (against focal point Z = 0).
The non-linear refractive properties of the ruthenium(II) complexes
were assessed by dividing the normalized Z-scan data obtained closed aperture
configuration by the normalized Z-scan data obtained under the open aperture
configuration, for example see Fig.3b. An increase in transmittance followed by a decrease
in transmittance (peak/valley) shows a negative nonlinear refraction.
The non-linear refractive index n2 can be derived from the
difference between normalized transmittance values at valley and peak positions (DTv-p)
by eqn. (3).
n2 = ![]() D Tv-p
(3)
D Tv-p
(3)
In accordance with the
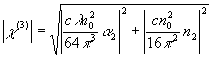 (4)
(4)The corresponding modulus of the hyperpolarizability g can be obtained by eqn. (5).
Where N is the number density of the solute in the solution (in cm-3), and n0 is the linear index of refraction of the complexes, in our experiment, the n0 of the all samples is 1.432.
It should be noted that the NLO parameters derived in this paper are regarded as effective parameters only. Both excited state absorption and two-photon absorption can be responsible for the measured NLO effects. The existing experimental data are insufficient to allow identification of the relative contributions of these two mechanisms. Compared with some known NLO chromophores (9.42¡Á10-32 esu for an planar open structure [Et4N]2[WS4Cu4(SCN)4(SCN)4(2- methylpyridine)4] at 532 nm[18], 2.27¡Á10-32 esu for a alkynylruthenium dendrimer at 800 nm[19]), the |g| values obtained for the new complexes are larger with reference to other known third-order NLO materials. The Z-scan results denote that the extended p delocalization of the diimine ligands exerts an effect on the third-order NLO properties of the complexes, and the values of |g| increase in the order: tatp < dppz < dppn. This can be attributed to the extension of the electronic p-system.
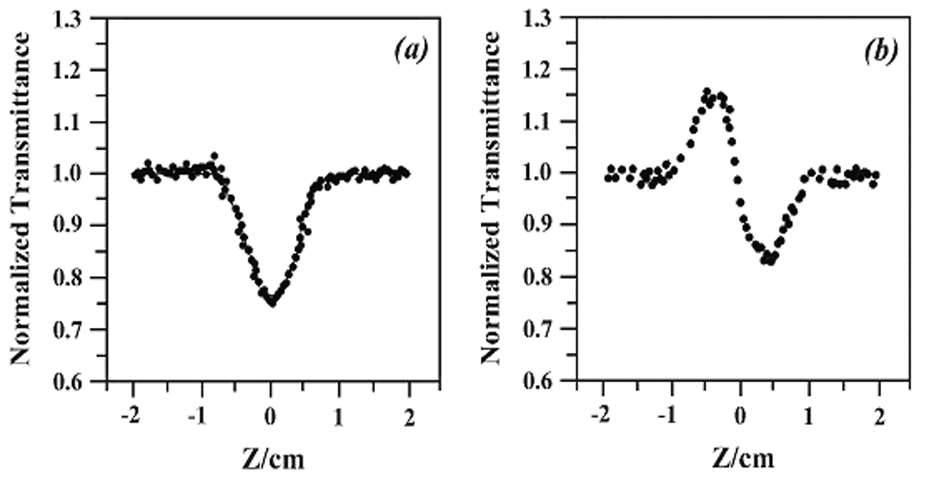
Figure 4 Z-scan data (filled circles) of 5¡Á10-5 mol dm-3 of [Ru(dppn)2(NOP)][ClO4]2 H2O, at 540 nm with I(Z = 0) being 2.33¡Á1012 W/m2: (a) collected under open aperture configuration showing NLO absorption. The solid curve is a theoretical fit based on eqs 1 and 2; (b) obtained by dividing the normalized Z-scan data obtained under closed aperture configuration by the normalized Z-scan data in (a). It shows self-defocusing effect of the complex.
4. CONCLUSIONS
Three new ruthenium (II) complexes containing extended diimine ligands have been
synthesized and characterized. The nonlinear optical properties of the ruthenium(II)
complexes were investigated by Z-scan techniques. Results indicate that the
extension of the p
framework of the ligands can enhance the NLO properties of the complexes.
REFERENCES
[1] Dose E V, Wilson L J. Inorg. Chem., 1978, 17: 1978.
[2] Paris J P, Brandt W W. J. Am. Chem. Soc., 1959, 81: 5001.
[3] Bozec H L, Renouard T. Eur. J. Chem. 200: 229.
[4] Zyss J, Dhenaut C, Chauvan T et al. Chem. Phys. Lett. 1993, 206: 409.
[5] Dias A R, Garcia M -H, Rodrignes J C et al. Angew. Chem. Int. Ed., 1999, 38: 366.
[6] McDonagh A M, Humphrey M G, Samoc M et al. J. Am. Chem. Soc., 1999, 121: 1405.
[7] Hidetomo A,Yasuhiko Y, Kazuhiro M. Jpn Pat., 05273616, 1993.
[8] Tykwinski R R, Gubler U, Martin R E et al. J. Phys. Chem. B 1998, 102: 4451.
[9] Frazier C C, S. Suha S, Chen W D et al. Polymer, 1987, 28: 503.
[10] Chao H, Li R H, Ye B H et al. J. Chem. Soc., Dalton Trans, 1999: 3711.
[11] Wu J Z, Li L, Zeng T X et al. Polyhedron, 1997, 16: 103.
[12] Lincoln P, Broo A, Norden B. J. Am. Chem. Soc., 1996, 118: 2644.
[13] J. P. Collin and J. P. Saurage. Inorg. Chem., 1986, 25: 135.
[14] Sullivan B P, Salmon D J, Meyer T J. Inorg. Chem., 1978, 17: 3334.
[15] Lin C T, Bottcher W, Chou M et al. J. Am. Chem. Soc., 1976, 98: 536.
[16] Hartshorn R M, Bartom J K. J. Am. Chem. Soc., 1992, 114: 5919.
[17] Amouyal E, Homsi A, Chambron J C et al. J. Chem. Soc., Dalton Trans, 1990: 841.
[18] Zhang C, Song Y L, Jin G C, Fang G Y et al. J. Chem. Soc., Dalton Trans, 2000: 1317.
[19] McDonagh A M, Humphrey M G, Samoc M et al. Organometallics, 1999, 18: 5195.
¡¡