http://www.chemistrymag.org/cji/2003/05c091ne.htm |
Dec. 1, 2003 Vol.5 No.12 P.91 Copyright |
High efficient blue light emitting pyridine-containing conjugated polymers prepared by oxidative-coupling copolymerization
Zheng Jinyun, Qin Jingui, Zhan Caimao
(College of Chemistry & Molecular Sciences, Wuhan University, Wuhan 430072)
Received Aug.11, 2003; Supported by the National Natural Science Foundation of China (No.20274031 ).
Abstract Two kinds of new
polymers incorporated pyridine with fluorene or triphenylamine moieties into main chain to
form main chain conjugated polymers have been prepared by oxidative-coupling
copolymerization. The polymers are all thermostable, highly soluble and have high quantum
yield. The fluorescence spectra of the polymers display blue light emitting properties.
Keyword pyridine, triphenylamine, fluorene, oxidative-coupling
copolymerization£¬conjugated polymers£¬blue light emitting
1£®INTRODUCTION
It is well known that the balanced charge injection from both electrodes and the
comparable mobility of electrons and holes within the polymers are crucial for achieving
high polymer light emitting device (PLED) efficiency. An attractive way to achieve high
efficiency in PLEDs is to develop new conjugated polymers with the desired injection
ability for electrons and/or holes. Polypyridine (PPy) is a moderately electron-deficient p system, which has excellent resistance
to photochemical and electrochemical oxidation, and has high thermal stability[1].
PPy has acted as an electron-injection material for organic light emitting device (LED)
and improved the properties of the device[2-3]. PPy is also a blue-green
emitter[4]. However, the efficiency of PPy single layer LEDs with ITO and
Aluminium contacts is very low, the external quantum efficiency is off order 0.001%[4-5].
A possible explanation for the low efficiency is that there is a large barrier to hole
injection from ITO into PPy[6]. Fujii A.[7] et al. synthesized
the alternating copolymer poly(2,5-dialkoxy-1,4-phenylene-alt-2,5-pyridine), which
improved the solubility of PPy. However, in order to preparate pyridine-based main chain
conjugated polymer, it is necessry to introduce bifunctional groups into the aromatic
rings of pyridine and the comonomers. In this communication, we incorporate the moieties
of triphenylamine, the well known hole transporting materials, into the polypyridine main
chain by facile direct polymerzation. The new copolymer (Polymer 1) may afford balanced
charge injection and higher quantum efficiency.
Polyfluorene (PF) and their derivatives have arisen special interest
because of their high efficiencies both in PL and EL[8-9]. Incorporating
fluorene moieties into PPy main chain may improve the efficiency of the polymer. In
addition, PFs show excimer and/or aggregate formation upon thermal annealing or the
passage of current. The structure of the alkyl substituents of PF influences the
solid-state packing of the polymers, especially the n-alkyl substituents[10].
At present we present a facile method of preparing the copolymer incorporated moieties of
unsubstituted fluorene and pyridine as main chain by oxidative-coupling copolymerization.
The copolymer (Polymer 2) may has high quantum yield and diminish aggregation or excimer.
2. EXPERIMENTAL SECTION
The copolymers were synthesized in good yield from readily available starting
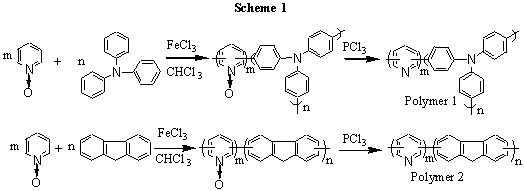
materials by oxidative-coupling copolymerization (showing in Scheme 1). The synthesis
method see reference [11]. The reaction times and yields are listed in Table 1.
3. RESULTS AND DISCUSSIONS
3.1 FT-IR Spectra
The FT-IR spectra of the polymers display bands around 3030, 1590 and 1487, and 810 cm-1
corresponding to the unsaturated C-H vibration, unsaturated -C=C- vibration and two
adjacent hydrogens out-of plane vibration of the benzene ring. In addition, Polymer 1
displays band at 1313 cm-1 corresponding to the saturated N-C stretching band
of triphenylamine. While Polymer 2 displays band at 2892 cm-1 due to the
saturated C-H vibration of fluorene.
3.2 1H NMR
In the 1H-NMR spectrum of Polymer 1 in CDCl3, all the signals
positioned at d=6.8 to 8.2 ppm were confirmed to the aromatic structure of the copolymer. The
signals at 8.191 ppm and 8.068 ppm were assigned to the hydrogens of pyridine. The 1H-NMR
spectrum of Polymer 2 displays two sets of lines originating in the 9-position protons of
fluorene around 4 ppm and in the aromatic proton between 7.032 and 7.922 ppm.
3.3 UV-Vis Spectra
The UV-Vis spectrum of Polymer 1 in THF
solution exhibits two almost equal strong absorption bands at 309.5 and 251 nm. Polymer 2
displays two absorption bands at 330.0 nm (a broad band) and 238.5 nm (a low sharp band).
In addition, the UV-Vis spectra of Polymer 1 and Polymer 2 in solid state film display
absorption bands at 316.5 and 301 nm respectively.
3.4 Solubility and Molecular Weight
The solubility of the polymers was measured and the results
were listed in Table 1, which reveals that the Polymer 1 has excellent solubility in
common organic solvents due to the high branched structure of the triphenylamine
copolymer. Polymer 2 is fairly soluble in common organic solvents.
The number-average molecular weight (Mn) and weight-average
molecular weight (Mw) of the Polymer 1, determined by size exclution
chromatography were 2.197¡Á104, and 3.795¡Á104, with the
polydispersity index of 1.73.
Table 1. The reaction, solubility and quantum yield of the polymers
Polymer |
Reaction times(h) | Yield (%) |
Solubility (g/100 mL) |
Quantum yield | ||||
CHCl3 |
toluene |
THF |
||||||
Polymer 1 |
40 |
47.3 |
4.15 |
3.05 |
2.66 |
0.350 |
||
Polymer 2 |
45 |
61.8 |
1.12 |
0.31 |
0.75 |
0.716 |
||
3.5 Morphology
The wide-angle X-ray scatting curves of Polymer 1 and Polymer 2 reveal that Polymer 1 is
completely amorphous, while Polymer 2 is slightly crystalline, which may ascertain the
high branched structure of the Polymer 1.
3.6 Thermolstability
All the polymers have high thermal stability. Thermogravimetric analysis indicated that no
thermal degradation of polymers takes place below 320
3.7 Fluorescence Spectra
The fluorescence spectra of the copolymers in solid state film show that they are blue light emitting materials. Polymer 1 (Figure 1.) displays very similar emission both in chloroform solution and in solid film with the maximum at 460 nm. Polymer 2 (Figure 2.) displays relatively short emission in chloroform solution with the maximum at 382 nm, while the emission in solid state film is very sharp and narrow with the maximum at 457 nm, which is red shifted compared with that in solution.
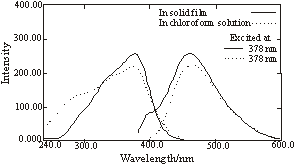
(Slitwidth: 3mm)
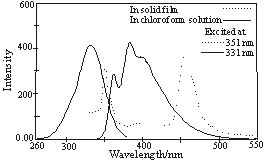
Figure 2 The fluorescence spectra of polymers 2 in chloroform solution and in solid film
(Slitwidth: 3mm)
The emission spectra of
Polymer 2 in chloroform solution with different concentrations were measured. The polymer
has the highest emission intensity with the concentration of 10-5 mol/L. Below
the concentration of 10-5 mol/L, the emission intensity decreased with
decreasing concentration. In the higher concentration, concentration quenching was
observed, but no excimer observed in this condition.
The quantum yields of the polymers in THF solution were measured
relative to quinine sulphate. The quantum yields (absolute value) are listed in Table 1.
The polymers all have high quantum yields. The Polymer 2 has higher yields than Polymer 1,
which ascertains the point that the incorporation of fluorene moieties can improve the
efficiency of the polymers.
4. CONCLUSIONS
Two kinds of polymers incorporated pyridine, fluorene and triphenylamine moieties into
main chain have been prepared by oxidative-coupling copolymerization. The polymers are all
thermostable with the onset decomposition temperature above 310
REFERENCES
[1] Yamamoto T, Maruyama T, Zhou Z et al. J. Am. Chem. Soc., 1994, 116 (11): 4832.
[2] Dailey S, Monkman A P, Samuel I D W. Synth. Met., 1999
[3] Dailey S, Halim M, Rebourt E et al. J. Phys.: Condens. Mater., 1998, 10: 5171.
[4] Gebler D D, Wang Y Z, Blatchford J W et al. J. Appl. Phys., 1995, 78 (6): 4264.
[5] Monkman A P, Halim M, Dailey S et al. SPIE Proc., 1997, 3145: 208.
[6] Dailey S, Halim M, Rebourt E et al. SPIE., 1997, 3148: 82.
[7] Fujii A, Ootake R, Fujisawa T et al. Appl. Phys. Lett., 2000, 77 (5): 660.
[8] Grice A W, Bradley D D C, Bernius M T et al. Appl. Phys. Lett., 1998, 73 (5): 629.
[9] Janietz S, Bradley D D C, Grell M et al. Appl. Phys. Lett., 1998, 73 (17): 2453.
[10] Scherf U, List E J W. Adv. Mater., 2002, 14 (7): 477.
[11] Zheng J Y, Zhan C M, Qin J G et al. Chem. Lett. 2002, 1222.
¡¡
¡¡¡¡