http://www.chemistrymag.org/cji/2004/061009pe.htm |
Jan.12, 2004 Vol.6 No.1 P.9 Copyright |
Min Xinmin, Xing Xueling, Hong Hanlie
(State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070) Received Nov. 22, 2003; Supported by the National Natural Science Foundation of China (20271040).
Abstract The relation between electronic
structure, chemical bond and thermoelectric property of Ca3Co2O6
and Ca3Co4O9 is studied by using density function
and discrete variation method(DFT-DVM). The gaps between the highest valence band (HVB)
and the lowest conduction band(LCB) of Ca3Co2O6 and Ca3Co4O9
both show the semiconductor property, and the gap of Ca3Co4O9
is less than that of Ca3Co2O6. Co 3d and O 2p mainly
contribute to HVB and LCB near Fermi level, but there is obvious difference between Ca3Co2O6
and Ca3Co4O9. The Co-O covalent and ionic bonds of Ca3Co4O9
are both stronger than those of Ca3Co2O6. Ca plays the
role of connections between the chains of ...... -Co(1)O3-Co(2)O3-
...... in Ca3Co2O6, and
between CoO2 and Ca2CoO3 layers in Ca3Co4O9.
The component of Ca also can decrease ionic and covalent bond strength and prove the
thermoelectric property.
Keywords cobaltite, electronic structure, chemical bond, thermoelectric property
1 INTRODUCTION
In general, the thermoelectric performance of oxide compounds is believed to be much
lower than that of the semiconductor alloys because of their high ionic character that
generally causes strong localization of electrons and thus lead to very low carrier
mobility. However, it has recently reported that NaxCo2O4
has a much large thermoelectric power[1], which is almost
comparable to that of the conventional thermoelectric materials. The conventional
thermoelectric materials, such as Bi2Te3, PbTe and SiGe, have some
inevitable shortages. For example, they are not stable in air at high temperature, toxic
to environment and difficult to manufacture. Oxides can overcome the shortages as
thermoelectric materials, so they have attracted much attention. Ca3Co2O6
and Ca3Co4O9 are the stable phases in the Ca-Co-O system.
However, understanding of electronic structure of these technologically important
cobaltite oxides has quite limited. Most recently, Asahi et al have calculated the
band-structure of one of the cobaltite oxides, i.e., (Ca2CoO3)4(CoO2)6[2],
but the other cobaltite oxides have not been reported. In this paper, the electronic
structures of Ca3Co2O6 and Ca3Co4O9
are calculated using density function and discrete variation method(DFT-DVM). The
relations between electronic structure, chemical bond and thermoelectric property are
discussed.
2 CALCULATION DETAILS AND MODEL
DFT-DVM method[3] is used to resolve the Kohn-Sham equation. A number of
discrete sampling points in a three-dimensional grid are chosen. The parameter in the
error function is varied to obtain all the minima for the points. Using the
multi-dimensional numeral integer method, self-consistent process is carried out to obtain
energy eigenvalue, wave function and others. DFT-DVM method can be used to calculate
larger structure models of molecules, clusters and solids, so it has been widely used in
chemistry, physics, material science an so on[3,4].
The crystal structure of Ca3Co2O6
belongs to space group![]() [5].
Its crystal cell is shown in Fig.1. The cobalt atoms are divided into two types, Co(1) and
Co(2). The framework of the cell is a cube(Fig.1 a). One Co(1) is in the center, and other
eight Co(1)'s are at the corners. One Co(1) atom at the corner is computed as 1/8 based on
the principle of corner-sharing in crystal theory. Therefore, there are two Co(1) atoms in
the cell. A Co(1)-Co(2)-Co(1)-Co(2)-Co(1) chain is along the diagonal, so there are two
Co(2) atoms in the cell. Co(1) in the center is coordinated by 6 O(1) atoms. The 6 O(1)
atoms are divided into two classes, and they coordinate to two Co(2) atoms above and below
the center Co(1) respectively. Therefore, the chain along the diagonal consist of
alternating face-sharing [Co(1)O6] octahedron and [Co(2)O6] trigonal
prisms and can be shown as -Co(1)O3-Co(2)O3- ... chain. Ca atoms are at the positions among the chains. There is a
O(2) atom near to every Co(1) at the corner in the cell. 22 atoms, i.e. 4 Co, 6 Ca and 12
O, exit in the cell. To revolve the chain as the vertical axis, the hexagon projection of
atoms in the cell is shown in Fig.1b. Atoms of Co(1)-Co(2)-Co(1)-Co(2)-Co(1) chain all are
projected in the center. Other 6 Co(1) atoms are at the corner of the hexagon, but they
are not in the same height. In reality, with the original point of Co(1) in the center,
the 6 Co(1) atoms at the corner of the hexagon are with symmetry of D3d point
group. As the same, 6 Ca atoms, or 6 O(1) and 6 O(2), are also with symmetry of D3d
point group, respectively. The calculated model of Ca3Co2O6
is expanded into 66 atoms, or [Ca18Co12O36], which is
three times as the cell. The 22 atoms discussed above are the insides of the model, and
other 44 atoms are the outsides. These 66 atoms are divided into 26 classes with the
symmetry operation of C3 point group.
[5].
Its crystal cell is shown in Fig.1. The cobalt atoms are divided into two types, Co(1) and
Co(2). The framework of the cell is a cube(Fig.1 a). One Co(1) is in the center, and other
eight Co(1)'s are at the corners. One Co(1) atom at the corner is computed as 1/8 based on
the principle of corner-sharing in crystal theory. Therefore, there are two Co(1) atoms in
the cell. A Co(1)-Co(2)-Co(1)-Co(2)-Co(1) chain is along the diagonal, so there are two
Co(2) atoms in the cell. Co(1) in the center is coordinated by 6 O(1) atoms. The 6 O(1)
atoms are divided into two classes, and they coordinate to two Co(2) atoms above and below
the center Co(1) respectively. Therefore, the chain along the diagonal consist of
alternating face-sharing [Co(1)O6] octahedron and [Co(2)O6] trigonal
prisms and can be shown as -Co(1)O3-Co(2)O3- ... chain. Ca atoms are at the positions among the chains. There is a
O(2) atom near to every Co(1) at the corner in the cell. 22 atoms, i.e. 4 Co, 6 Ca and 12
O, exit in the cell. To revolve the chain as the vertical axis, the hexagon projection of
atoms in the cell is shown in Fig.1b. Atoms of Co(1)-Co(2)-Co(1)-Co(2)-Co(1) chain all are
projected in the center. Other 6 Co(1) atoms are at the corner of the hexagon, but they
are not in the same height. In reality, with the original point of Co(1) in the center,
the 6 Co(1) atoms at the corner of the hexagon are with symmetry of D3d point
group. As the same, 6 Ca atoms, or 6 O(1) and 6 O(2), are also with symmetry of D3d
point group, respectively. The calculated model of Ca3Co2O6
is expanded into 66 atoms, or [Ca18Co12O36], which is
three times as the cell. The 22 atoms discussed above are the insides of the model, and
other 44 atoms are the outsides. These 66 atoms are divided into 26 classes with the
symmetry operation of C3 point group.
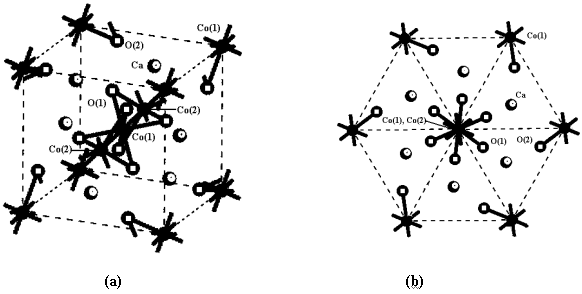
Fig.1 Crystal cell of Ca3Co2O6.
(a) The cube framework; (b) the projection of hexagon
Ca3Co4O9
is with symmetry of space group Cm[6].
Ca3Co4O9 has two layers structure, Ca2CoO3
in the center and CoO2 at the top and below as shown in Fig.2. The cobalt atoms
are also divided into two types, Co(1) in the CoO2 layer and Co(2) in the Ca2CoO3
layer. The oxygen atoms are divided into two types and belong to the two layers
respectively, too. The character structure of CoO2 layer is edge-sharing
[Co(1)O6] octahedron. Ca2CoO3 layer is similar to
structure of NaCl. There are normal a and c constants of Ca3Co4O9.
However, there is different cell constant along b axis (right and left direction in
Fig.2), b1(CoO2)=0.28238nm and b2(Ca2CoO3)=0.45582nm.
CoO2 or Ca2CoO3 layer is alone with space group Cm.
In the symmetry meaning, Ca3Co4O9 is much like a mixture
compound. The calculated model consists of the atoms of the right plane in Fig.2 as the
center part and the neighboring atoms. Therefore, the model is with 68 atoms, or [Ca14Co15O39]
and divided into 46 classes with symmetry operation of Cs point group.
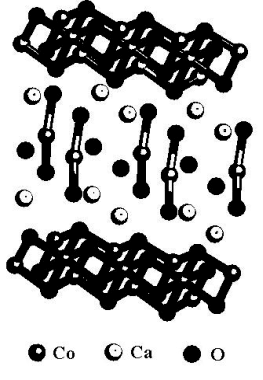
Fig.2 Structure of Ca3Co4O9
3 RESULTS AND DISCUSSION
The average values of the covalent bond orders between inside atoms are in Table 1.
The covalent bond of Co(1)-O is stronger than that of Co(2)-O in both of Ca3Co2O6
and Ca3Co4O9, but the strength difference between Co(1)-O
and Co(2)-O bonds in Ca3Co4O9 is more obvious than that
in Ca3Co2O6. On the other hand, the covalent bond of Co-O
in Ca3Co4O9 is stronger than that in Ca3Co2O6.
The average values of net charge of the inside atoms are also in Table
1. The charge of Co(1) is higher than that of Co(2) in both of Ca3Co2O6
and Ca3Co4O9, but the charge difference between Co(1) and
Co(2) atoms in Ca3Co4O9 is also more obvious than that in
Ca3Co2O6. On the other hand, the average value of charge
of Co(1) and Co(2) in Ca3Co4O9 is slightly higher than
that in Ca3Co2O6. The charge of Ca in Ca3Co4O9
is also higher than that in Ca3Co2O6. There are more
oxygen atoms with larger electronegtivity in Ca3Co4O9
than in Ca3Co2O6, which lead to the higher charge of
metal atoms in Ca3Co4O9. As there are less differences of
the distance between atoms between Ca3Co4O9 and Ca3Co2O6,
the Co-O ionic bonds of Ca3Co4O9 are also stronger than
those of Ca3Co2O6.
Table 1 Calculated covalent bond orders and atomic net charges
covalent bond orders |
atomic net charges |
|||||
Co(1)-O |
Co(2)-O |
Ca-O |
Co(1) |
Co(2) |
Ca |
|
Ca3Co2O6 |
0.135 |
0.108 |
0.085 |
1.033 |
0.910 |
0.870 |
Ca3Co4O9 |
0.199 |
0.161 |
0.067 |
1.188 |
0.770 |
1.349 |
The bonding model and
strength are further studied by the contour map of one electron wave function, or
molecular orbital. The maps of the representative orbitals of Co(1) and Co(2) of Ca3Co2O6
are shown in Fig.3 a and b respectively. Solid and dash lines express positive and
negative values of the molecular wave functions attributed from the atoms, respectively.
It has been pointed out that the original point of Co(1) is in the center, and its
first-neighboring 6 atoms of O(1) are with symmetry of D3d point group.
Therefore, the section of the Fig.3 a can be chosen to pass through the O(1)-Co(1)-O(1)
straight line in the center. On the other hand, with the original point of Co(2) in the
center, the first-neighboring atoms are 3 O(1) and 3 O(2) and only with symmetry of D3
point group. Therefore, O(1)-Co(2)-O(2) is not a straight line. The values of the out
contour lines between Co and O are indicated, and the values of Fig.3 a are larger than
that of b. Therefore, the strength of covalent bond of Co(1)-O is stronger than that of
Co(2)-O in Ca3Co2O6.
The maps of the representative orbitals of Co(1) and Co(2) of Ca3Co4O9
are shown in Fig.4 a and b respectively. Oxygen at below is in the section but that at the
top is above the section, so the O-Co-O from top to below is not a real straight line. It
is shown from comparing the values of the out contour lines between Co and O that the
strength of covalent bond of Co(1)-O is stronger than that of Co(2)-O in Ca3Co4O9.
Comparing Fig.3 and 4, it is indicated that Co-O covalent bonds of Ca3Co4O9
are stronger than those of Ca3Co2O6, which is consistent
with the above numeral results.
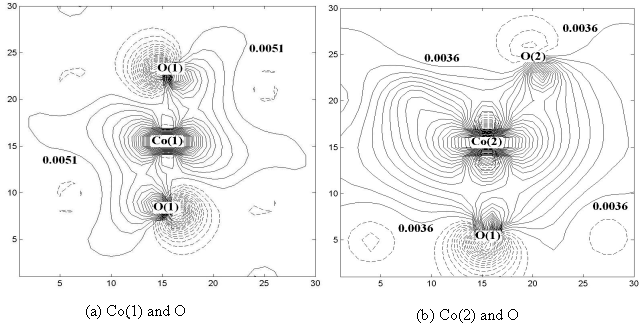
Fig.3 A pair of contour maps of the representative orbitals of Ca3Co2O6
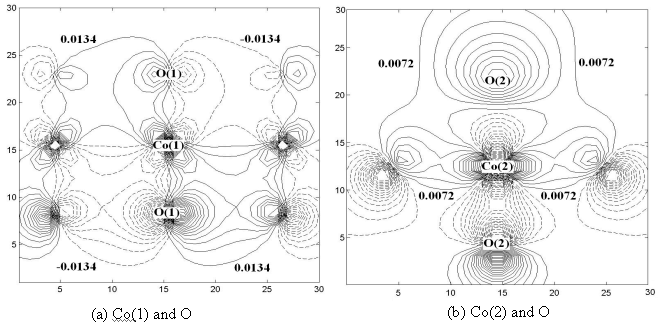
Fig.4 A pair of contour maps of the representative orbitals of Ca3Co4O9
The total density of state
(DOS), Co(1) 3d, Co(2) 3d, O(1) 2p and O(2) 2p partial DOS of Ca3Co2O6
are in Fig.5 a to e, respectively. The partial DOS is that of inside atoms (Co(1) is only
one in the center). Fermi level is at the zero point in the cross axis. The maximum of
total DOS is 100(%), and the contribution of partial DOS to the total DOS can be seen from
the vertical axis. DOS of other valent orbital, such as Co 4s, Ca 4s or O 2s, is rather
small in this energy field and not shown. In the total DOS, the gap between the highest
valence band (HVB) and the lowest conduction band (LCB) shows the semiconductor
characteristics. It is indicated from comparing the partial DOS that the contribution of
Co(1) 3d to HVB is larger than that of Co(2) 3d, but the contribution of Co(1) 3d to LCB
is less than that of Co(2) 3d. O 2p mainly contributes to HVB, and the peaks exist in the
wider field. There is less difference between O(1) and O(2) 2p contribution to HVB, but
the contribution of O(1) 2p to LCB is obviously nearer to Fermi level than that of O(2)
2p. The contributions of Co 3d to HVB and LCB nearer to Fermi level are larger than those
of O 2p, which is in agreement with Asahi et al.'s conclusion in the calculation of (Ca2CoO3)4(CoO2)6[2].
The n-type transport is primarily through Co(2) 3d orbitals (contributing to LCB) and
p-type transport through Co(1) 3d orbitals (contributing to HVB).
The total, Co(1) 3d, Co(2) 3d, O(1) 2p and O(2) 2p partial DOS of Ca3Co4O9
are also shown in Fig.6 a to e, respectively. Co 4s, Ca 4s or O 2s, is also rather small
in this energy field and not shown. The gap between HVB and LCB of total DOS of [Ca18Ni3Co9O36]
also shows the semiconductor characteristics. The gap of Ca3Co4O9
is less than that of Ca3Co2O6, and it results from the
obvious difference between the electron structures of two systems and should affect
electrical conductivity and thermoelectric property. It is indicated from comparing the
partial DOS that the main peaks of Co(1) 3d states exist in both of HVB and LCB, but the
peaks are some far away from Fermi level. Co(2) 3d states are mainly contribution to LCB
and near to Fermi level. The main peaks of O(1) 2p states also exist in both of HVB and
LCB, but the peaks are further away from Fermi level. The HVB near Fermi level is mainly
the contribution from O(2) 2p states.
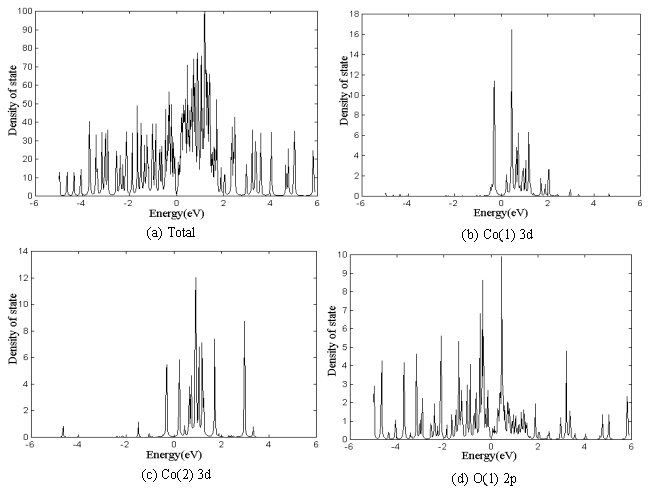
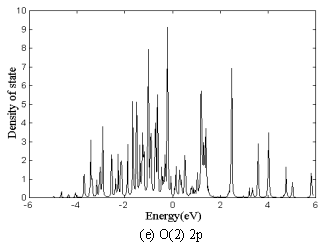
Fig.5 DOS of Ca3Co2O6
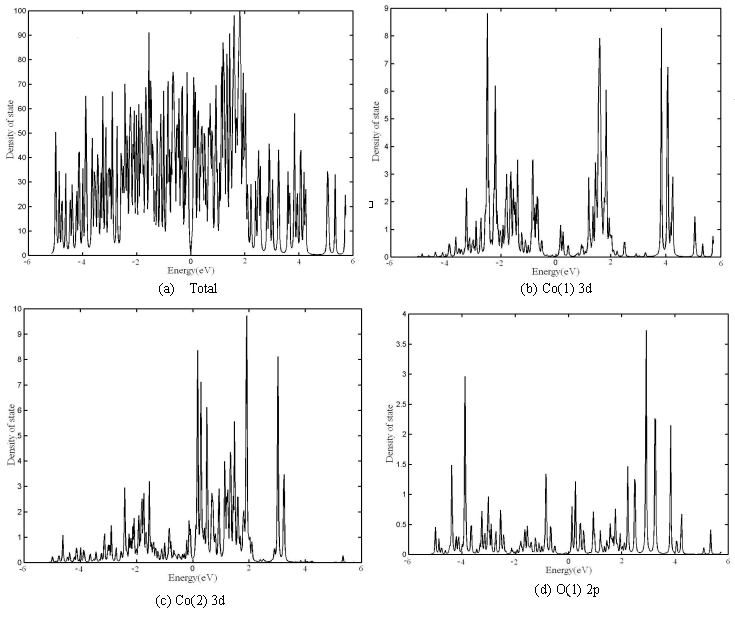
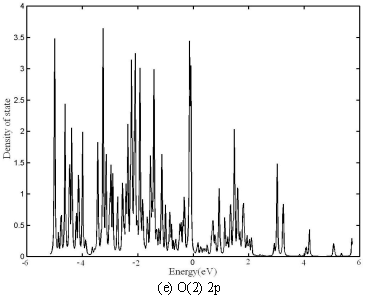
Fig.6 DOS of Ca3Co4O9
The contributions to HVB
and LCB near Fermi level in Ca3Co2O6 are mainly from O(1)
and O(2) 2p, and Co(1) and Co(2) 3d, but those in Ca3Co4O9
are only mainly from O(2) 2p and Co(2) 3d, which is also an obvious difference of partial
DOS between Ca3Co2O6 and Ca3Co4O9.
Therefore, the semiconductor, or thermoelectric property of Ca3Co4O9
should be mainly from Ca2CoO3 layer, but it seems to have no direct
relation to the CoO2 layer. It is well known that binary oxides (such as Co2O3)
hardly have thermoelectric property, but trinary oxide compounds (such as Ca3Co2O6
and Ca3Co4O9) have quite good thermoelectric property,
which is consistent with our conclusion. Binary oxides often have high ionic or covalent
character, such CoO2 layer in Ca3Co4O9 (Table
1), so hardly have good thermoelectric property. Co-O ionic and covalent bonds of Ca3Co2O6
and Ca2CoO3 layer in Ca3Co4O9 are
all weaker than those of CoO2 layer in Ca3Co4O9,
and the component Ca can decrease ionic and covalent bond strength.
In Ca3Co4O9 the first-neighboring 6
oxygen atoms to Co(1) are all in the CoO2 layer, and the first-neighboring 8
oxygen atoms to Co(2) are all in the Ca2CoO3 layer. In other word,
there is no direct interaction between Co and O in the different layers. On the other
hand, the first-neighboring 8 oxygen atoms to Ca are in both of CoO2 and Ca2CoO3
layers. Therefore, Ca plays a role to connect interaction between CoO2 and Ca2CoO3
layers. In Ca3Co2O6 the first-neighboring 6 oxygen atoms
to Co(1) are all O(1), and those to Co(2) are 3 O(1) and 3 O(2). The oxygen atoms
coordinate to both Co(1) and Co(2) that neighbors each other are in the same -Co(1)O3-Co(2)O3-
... chain. Therefore, there is no direct interaction between
Co and O in the different chains. The first-neighboring 8 oxygen atoms to Ca are O(1) and
O(2), and these oxygen atoms coordinate several cobalt atoms in the different chains. Ca
also plays a role to connect interaction among ... -Co(1)O3-Co(2)O3- ... chains.
4 CONCLUSIONS
The gaps between the highest valence band (HVB) and the lowest conduction band(LCB) of
density of state of Ca3Co2O6 and Ca3Co4O9
both show the semiconductor property, and the gap of Ca3Co4O9
is less than that of Ca3Co2O6.
The contributions to HVB and LCB near Fermi level in Ca3Co2O6
are mainly from O(1) and O(2) 2p, and Co(1) and Co(2) 3d, but those in Ca3Co4O9
are only mainly from O(2) 2p and Co(2) 3d in Ca2CoO3 layer.
Therefore, the semiconductor, or thermoelectric property of Ca3Co4O9
should be mainly from Ca2CoO3 layer, which is consistent with that
binary oxides hardly have thermoelectric property, but trinary oxide compounds have quite
good thermoelectric property.
CoO2 layer in Ca3Co4O9 has
high ionic and covalent character. Ionic and covalent bonds of Ca3Co2O6
and Ca2CoO3 layer in Ca3Co4O9 are
all weaker than those of CoO2 layer in Ca3Co4O9.
The Co-O covalent and ionic bonds of Ca3Co4O9 are both
stronger than those of Ca3Co2O6.
Ca plays the role to connect the interaction among ...-Co(1)O3-Co(2)O3-
... chains in Ca3Co2O6, and to connect the interaction
between CoO2 and Ca2CoO3 layers in Ca3Co4O9.
The component of Ca also can decrease ionic and covalent bond strength and prove the
thermoelectric property.
RERERENCES
[1] Sasago T Y, Uchinokura K. Phys. Rev.
B, 1997, 56: 12685.
[2] Asahi R, Sungiyama J, Tani T. Phys. Rev.
B, 2002, 66: 1551031.
[3] Xiao Shenxiou, Wang Congyu and Chen Tianlang. The Method of Density Function and
Discrete Variation Used in Chemistry and Material Physics. Beijing: Science Press. 1998.
[4] Min Xinmin, Hong Hanlie, An Jiming, J. Wuhan Uni. Tech. Mat. Sci. Ed., 2002, 17: 6.
[5] Helmer F, Egil G, Siv A et al. J. Solid State Chem., 1996, 124: 190.
[6] Miyazaki Y, Onoda M, Oku T et al. J. Phys. Soc. Japan. 2002, 71: 491.