http://www.chemistrymag.org/cji/2004/066039ne.htm |
Jun. 1,
2004 Vol.6 No.6 P.39 Copyright |
Liu Hanxing, Wang Xian, Hu Weida , Guo
Liling , Ouyang Shixi
(State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan
University of Technology, Wuhan 430070, China)
Received Mar. 8, 2004; Supported by Key Program of the National Nature Science Foundation of China (No.90206047)
Abstract
Organic-inorganic layered perovskite-type compounds (C4H9NH3)2CuX4
(X=Cl, Br) were prepared from solutions in an air atmosphere. X-ray diffraction, Scanning
electron microscopy (SEM), FTIR spectroscopy and thermal analysis (TG and DSC) were used
to characterize the obtained powders. From the SEM pictures, both of (C4H9NH3)2CuCl4
and (C4H9NH3)2CuBr4 took on
obvious sheet-like microstructure, as well as their XRD profiles demonstrated the crystals growth were highly oriented. The FTIR spectrum showed that the characteristic
peaks of (C4H9NH3)2CuCl4 are
shifted to higher frequency due to protonation of C4H9NH2.
The thermal stability of (C4H9NH3)2CuBr4
was inferior to (C4H9NH3)2CuCl4 due
to its higher Jahn-Teller distortion of inorganic sheets.
Keywords layered
perovskite-type, characterization, microstructure, thermal stability
1. INTRODUCTION
Layered organic-inorganic perovskite-type materials have been studied intensively due
to their potential for unique electrical, magnetic, and optical properties. In these
system, organic and inorganic components combined into a molecular material. Organic
components provide virtually unlimited flexibility to choose molecules of varying length,
width, polarizability, and degree of saturation or polymerization, as well as offering
high luminescent properties, large polarizability, plastic mechanical properties, ease of
processing, and structure diversity. Inorganic components, on the other hand, offer the
potential for a wide range of electronic properties (enabling the design of insulators,
semiconductors, and metals), magnetic and dielectric transitions, substantial mechanical
hardness and thermal stability [1-5]. The (RNH3)2MX4
(M=divalent metal, X=halogen) members of the perovskite-type family have been synthesized
from solution and have generated considerable interest as self-assembling
multi-quantum-well structures, where inorganic layers of two-dimensional network of
corner-sharing octahedral metal halide MX6 and organic layer of molecular
ammonium cation RNH3 are alternately piled up, R can be substituted by alkyl
group, chromophore, etc [6,7]. Due to the low dimensionality of the inorganic
sheets, the exciton has a large binding energy of several millielectron volts, which
enables strong photoluminescence even at room temperature sufficient to offer potential
applications in emitter materials in electroluminescent devices[8-12]. In
this paper, we report the preparation of (C4H9NH3)2CuX4
(X=Cl, Br) by a solution method in an air atmosphere and characterize their layered
perovskite-type structures, furthermore test their thermal stabilities. The reason why we
choose the titled systems lies in two facts. Firstly, numerous papers have reported there
were phase transitions in the layered (RNH3)2CuCl4
systems and caused which properties changing at different temperatures, the prepared new
crystals of butylammonium tetrahalocuprate (C4H9NH3)2CuX4
should be considered as two-dimensional materials according to the butyl chain length, in
the future work, we would also study their electronic and magnetic properties in
comparison with the analogous systems (RNH3)2CuCl4
reported in the publication papers [8,11,13]. Secondly, the differences of
crystal structure and thermal stability between the titled materials can help us to
conclude the effects on the compound due to different halogen.
2. EXPERIMENTAL
2.1 Sample preparation
Crystal of the title compounds were grown from slowly cooled aqueous HCl acid solution
in the air. The compound (C4H9NH3)2CuCl4
was synthesized by first dissolving 6.8192g (0.04mol) of CuCl2·2H2O
in 25ml aqueous HCl (37%wt) at 70ºC in a water bath, and 7.9072ml (0.08mol) of C4H9NH2
in 6.6246ml (0.08mol) aqueous HCl (37%wt) to protonation. Upon dropwise addition of the
organic ammonium solution into the CuCl2 solution, drastically reaction began
and green precipitate immediately formed. The solution was allowed to cool and filtered at
decompression. Then the green powders turned to yellow and was dried in vacuum dry box at
70ºC.
Crystals of (C4H9NH3)2CuBr4
was prepared by a method analogous to the X=Cl. Briefly, 6.7011g (0.03mol) of CuBr2
was dissolved in 30ml aqueous HBr (40%wt) at 70ºC in a conical flask, and 5.9303ml (0.06mol) of C4H9NH2
in 8.7946ml (0.06mol) aqueous HBr (40%wt) in a separate beaker and then added to CuBr2
solution. After cooling the solution, the purple crystals formed was filtered and dried in
vacuum at 70ºC.
Element analysis for the two products was taken by Elementar
Analysensysteme GmbH VarioEL III, the found and [theoretical] results are illustrated in
Table 1. It can be seen the found value is close to the proposed C8H24N2CuX4
formula.
Table 1. The C, H, N content of (C4H9NH3)2CuX4
(X=Cl, Br)
Sample |
(C4H9NH3)2CuCl4 |
(C4H9NH3)2CuBr4 |
|
|
C |
27.37 [27.17] |
18.06 [18.08] |
H |
6.520 [6.84] |
4.476 [4.55] |
|
N |
7.779 [7.92] |
4.975 [5.27] |
|
2.2 Characterization
The crystallinity and orientation of the powder samples were determined by X-ray
diffraction. X-ray diffraction (XRD) measurements recorded with a 2q
3. RESULTS AND DISCUSSION
3.1 X-ray diffraction
¡¡
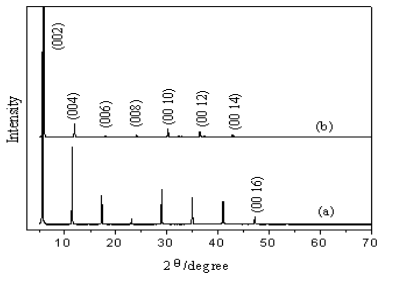
Fig.1 XRD profiles for powder of (C4H9NH3)2CuCl4 (a) and (C4H9NH3)2CuBr4 (b)
3.2 Scanning electron microscopy
Fig.2 shows SEM pictures of powders of (a) (C4H9NH3)2CuCl4
and (b) (C4H9NH3)2CuBr4.It can be
seen from Fig.2 that the two samples take on obvious sheet-like microstructures. We have
known that organic-inorganic layered perovskite-type assembling materials A2MX4
derive from three-dimensional perovskite structure, where M is a divalent metal, X is a
halide and A can be a wide range of organic amine. As shown in Fig.3, at the molecular
level, the 2-D inorganic MX42- layer and organic ammonium layer are
alternately stacked along c-axis
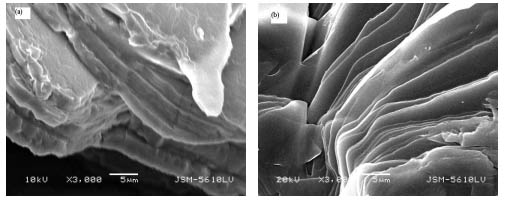
Fig.2 SEM micrographs of sample (C4H9NH3)2CuCl4 (a) and (C4H9NH3)2CuBr4 (b)
3.3 Infrared transmission analysis
Fig.4 shows FTIR spectrum of (a) C4H9NH2 and (b) (C4H9NH3)2CuCl4.
It can be seen from Fig.4 that the intensity and shape of the characteristic peaks exhibit
obvious differences between the reactant and precipitate. The characteristic peaks in the
compound include approximately 3439 cm-1, 3168 cm-1, 3131 cm-1,
1575 cm-1 and 1487 cm-1, which are broadened and intensified in
comparison to the free amine, moreover, the peaks are shifted to higher frequency (as well
as in the (C4H9NH3)2CuBr4). These
variations presumably ascribe to protonation of C4H9NH2
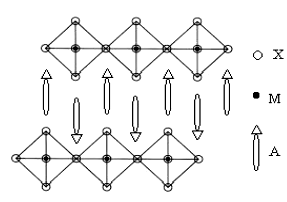
Fig.3 Schematic structure of layered perovskite-type A2MX4
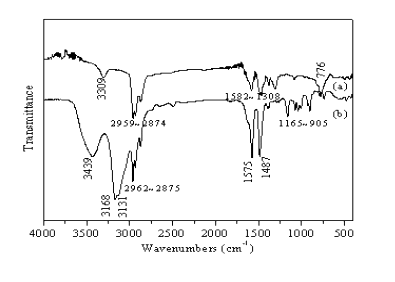
Fig.4 FTIR spectrum for C4H9NH2 (a) and (C4H9NH3)2CuCl4 (b) 3.4 Thermal analysis
The results of TG-DSC shows the decomposition of (C4H9NH3)2CuCl4 occurs at 261.6ºC, and (C4H9NH3)2CuBr4 at 197.0ºC respectively. The thermal stability of (C4H9NH3)2CuCl4 is obviously higher than that of (C4H9NH3)2CuBr4. According to the weight loss curve, we assume that gradual heating of (C4H9NH3)2CuX4(X=Cl, Br) compounds leads to the two step dissociation of the organic component from the structure:
(C4H9NH3)2CuX4 (s)¡úC4H9NH3X (g) + C4H9NH3CuX3 (s) ¡ú2C4H9NH3X (g) + CuX2(s) [15].
Because of the Jahn-Teller effect on [CuX6] inorganic sheets in (C4H9NH3)2CuX4, the N¨DH¡X hydrogen binding is weakened and the electrostatic repulsion is increased between the halide ion in the CuX42- anions [13]. We consider the dissociation of (C4H9NH3)2CuBr4 to C4H9NH3Br is easier than C4H9NH3Cl from (C4H9NH3)2CuCl4, presumably due to higher deformation of which inorganic sheets.
4. CONCLUSIONS
In air ambient, the layered perovskite-type powders of (C4H9NH3)2CuX4
(X=Cl, Br) can be acquired using a solution technique. The obtained crystals had high
orientation and their microstructures took on obvious sheet-like. The FTIR spectrum show
us the amine is protonation in the compound, which is in agreement with theoretical
assumption. The crystal structure and thermal properties of (C4H9NH3)2CuX4
(X=Cl, Br) has some differences due to the sort of halide anion, as we note that (C4H9NH3)2CuBr4
has less d-spacing value and lower decomposition point than (C4H9NH3)2CuCl4.
For the compounds (C4H9NH3)2CuX4
(X=Cl, Br), there should be interesting electronic and magnetic properties ascribed to
their characteristic structures as other systems having been reported, and further study
would be continued.
REFERENCES
[1] David B M, Chondroudis K, Kagan C R. IBM J. RES.& DEV., 2001, 45 (1): 29.
[2] Masanao Era et al. Thin Solid Films, 2001, 393: 24.
[3] David B M. Chem. Mater., 1996, 8: 79.
[4] Fujita T, Nakashima H, Hirasawa M et al. Journal of Luminescence, 2000, 87: 847.
[5] Etienne Wortham et al. Physical B, 2002, 318: 387.
[6] Braun M, Tuffentsammer W, Wachtel H et al. Chemical Physics Letters, 1999, 307: 373.
[7] Braun M, Tuffentsammer W, Wachtel H et al. Chemical Physics Letters, 1999, 303: 157.
[8] Salim Haddad, Roger D. Willett. Inorg. Chem., 2001, 40: 2457.
[9] Shigeki Kashiwamura, Nobuaki Kitazawa. Synthetic Metals, 1998, 96: 133.
[10] Papavassiliou G C, Mousdis G A, Koutselas I B. Synthetic Metals, 2001, 121: 1339.
[11] Eji Shikoh, Yasuo Ando, Masanao Era et al. Journal of Magnetism and Magnetic Materials, 2001, 226-230: 2021.
[12] Kazuhiro Sumioka, Hiroyuki Nagahama, Tetsuo Tsutsui. Applied Physics Letters, 2001, 78 (10): 1328.
[13] Taketoshi Sekine, Tsunehisa Okuno, Kunio Awaga. Chemical Physics Letters, 1996, 249: 201.
[14] David B M, K. Liang. Journal of solid state chemistry 134, 1997: 376.
[15] David B M et al. Chem. Mater., 1999, 11:542. ¡¡