http://www.chemistrymag.org/cji/2004/066041ne.htm |
Jun. 1,
2004 Vol.6 No.6 P.41 Copyright |
Optical emission spectrum online diagnoses for CH4+H2 discharge plasma system at atmospheric pressure
Ren Longliang, Cui Jinhua#
( Department of Physics, Science College, Tianjin University, Tianjin
300072; # Department of Chemistry, Liaoning Normal University, Dalian 116021 )
Keywords Plasma, CH4 coupling, optical emission spectrum diagnoses Spectral lines abound in optical spectrogram of plasma emission, from visible light to ultraviolet radiation and even to X-rays. The emission is caused by motion of numerous excited particles. Radiation of low temperature plasma mainly includes disexcited emission, compound emission and bremsstrahlung. There are spectral lines and bands in plasma emission [1]. In this study, it was found that spectral bands caused by electron transition of main existing species in the plasma emission were located in visible and ultraviolet regions. Spectral bands occur mainly because of vibration and rotation of valence electron transition in molecules, which is excited at 1-20eV of energy[2]. In terms of nature of radiation source, plasma belongs to gray-body[1] whereas highly dense plasmas produce black-body radiation[3]. The study dealt with highly dense plasmas at atmospheric pressure. Dagel et al [4] studied reaction kinetics of CH4 radio-frequency glow discharges at 30 m Torr. They believed that reaction rate was a function of CH4 consumption. They determined C2H6£¬C2H4£¬C2H2 and other species in the system with mass spectrograph. Here we show our work that the resultants in CH4 coupling system (CH4 : H2 =1 : 1 (mol), the same as the following) under plasmas at atmospheric pressure have been diagnosed online (300-700nm) in order to find out optimized parameters for CH4 coupling. The experiment set-up is as shown in Fig.1.
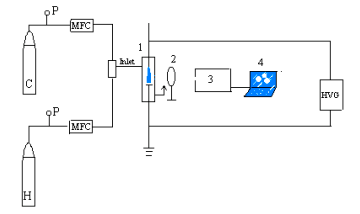
Fig.1 Emission spectrum diagnosis set-up for plasma methane coupling
C: CH4 tank, H: H2 tank, P: pressure gauge, MFC: mass flow controller, HVG: AC/DC high voltage generator, 1: reactor; 2: collecting lens (f=42mm, j=30mm), 3: WGD-3 grating spectrograph, 4: computer
1 CALIBRATION OF SPECTROGRAM
It is necessary to standardize WGD-3 spectrograph (made in Tianjin, China) used in the
experiments and calibrate the determined peak location of wavelength in the established
emission spectrum system with H2 standard discharge vessel and low-pressure Na
vapour standard discharge vessel.
1.1 H2 spectra
The results of calibration of the determined peak location of wavelength with standard
H2 discharge vessel are shown in Fig.2 according to the literature data in
Table 1.
Table 1 Characteristic lines of H-Balmer series
Spectral lines[5] |
Wavelength£¨nm£©[5] |
Transition[6] |
a1/2 [5] |
Ha |
656.2846 |
n = 3¡ú2 |
0.015 |
Hb |
486.1322 |
n = 4¡ú2 |
0.087 |
Hg |
434.0458 |
n = 5¡ú2 |
0.100 |
Hd |
410.1731 |
n = 6¡ú2 |
0.180 |
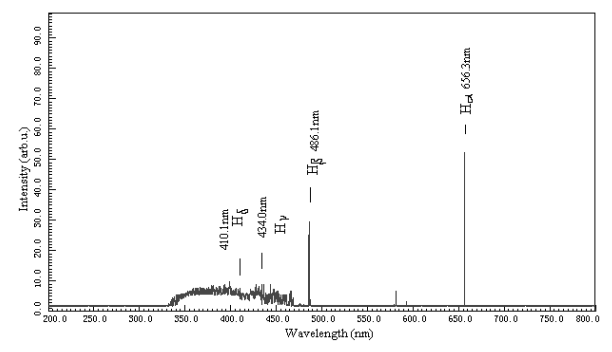
Fig. 2 Standardization of WGD-3
spectrograph with H2 standard discharge vessel
1.2 Low-pressure Na vapour standard
discharge vessel
According to literature [7]£¬characteristic
spectrum lines of low-pressure Na vapour standard discharge vessel are Na double lines,
respectively at wavelength of 588.99nm and 589.59nm.
The results of calibration with low-pressure Na vapour standard
discharge vessel for the WGD-3 spectrograph are as shown in Fig.3, which were well
coincident with the data of literature [7] just with ¡À0.017%of relative error.
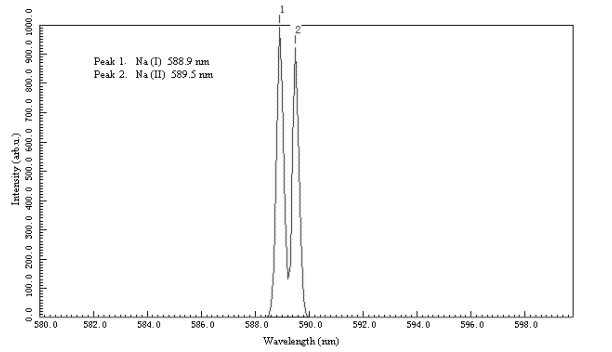
Fig. 3 Standardization of WGD-3 spectrograph with
low-pressure Na vapour standard discharge
vessel
2 RESULTS AND DISCUSSION
2.1 Optical emission spectrum online diagnoses for CH4
coupling under plasmas
As shown in Fig.4 (b)£¬72 W of supplied power was
applied to the CH4+H2 discharge plasma system with wire-plate
electrodes at atmospheric pressure whereas 50 W of that to the same in Fig.4 (a).
Black-body radiation and electrode sputtering were more violent in (b) because of higher
energy input of the electric field. C2 swan band in (b) was obviously weaker
than that in (a). It showed that C2 species decreased with the increase of
supplied power. It may be due to coke in CH4 coupling at excessive energy in
discharge channels. Data of the experimental results were compared with that in literature
as shown in Table 2. According to reason, CH and CH2 spectral band as well as C2
swan band should also occur in peak more weakly with the increase of supplied power,
but on the contrary, it was discovered in the experiments that CH, CH2 spectral
bands of CH4 + H2 discharge plasma emission occurred more strongly
with the increase of supplied power, As shown in Table 2 and Fig. 4. The reasons remained
to be studied. And it was observed that the most strong peak in C2 swan band
was 0-0 transition at 516.5 nm of wavelength.
As shown in Fig.4 and Table 2, it was observed in the experiments that
the electrodes spurted more violently with the increase of supplied power. The results
suggested that when the discharges at the higher supplied power, kinetic energy of cations
should increase with the increase of discharge power, i.e., discharge electric current
density and cathode potential. As a result of bombardment of the cations to the cathode,
locally high temperature on metal surface made atoms and molecules in metal spurt about,
i.e. cathode sputtering. And the higher the electric current density and cathode
potential, the more violent the cathode sputtering. When CH4 + H2
discharges in the electric fields were initiated with an AC high voltage generator, both
electrodes alternated as a cathode at certain frequency and both spurted.
The fact that copper electrodes spurted more violently in (b) than (a)
suggested that the mechanism of electrode sputtering should mainly be referred to as
cation bombardment at about 50 W of supplied power whereas at about 70 W, in addition to
having mechanism of cation bombardment in the discharges, metal evaporation should be
unavoidable since more violent electrode sputtering should cause higher temperature of the
system, especially of heavy particles. It was demonstrated that for electrode sputtering,
mechanism of metal evaporation in (b) is more than that of cation bombardment in (a). And
there was an obvious fact that a higher black-body radiation enveloping line occurred in
(b) rather than in (a). The result showed that temperature of heavy particles increased
with the increase of supplied power as in (b) and went up so closely to the temperature of electrons as to occur
more violently thermal movement of the particles.
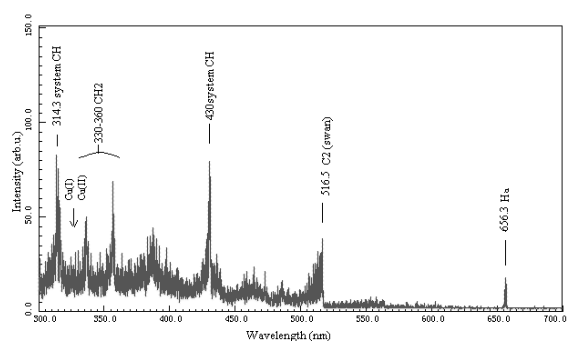
(a) Supplied power 50 W
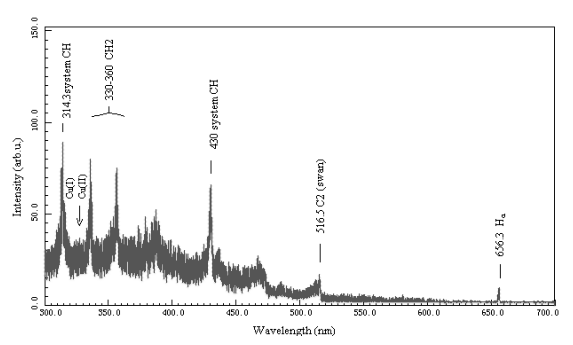
(b) Supplied power 72 W
Fig.4 Emission spectroscopy of CH4+H2 discharges at atmospheric
pressure
No. |
Species |
l m (detected, nm) |
l m (documents, nm) |
Transition |
1 |
CH |
(a) 313.7 (82.8 arb.u£© |
314.3 system [8] |
|
2 |
Cu£¨I£© |
(a) 324.7 (29.3 arb.u) |
324.7[9] |
0-30784cm-1 [9] |
3 |
Cu (II) |
(a)327.4 (18.8 arb.u) |
327.4[9] |
0-30535cm-1 [9] |
4 |
CH2 |
(a) 330-360 |
330-360 |
|
5 |
CH |
(a) 389.9 (38.6 arb.u) |
390 system [8] |
|
6 |
CH |
(a) 430.3 (65.9 arb.u) |
430 system [8] |
|
7 |
C2 (swan band) |
(a) 515.3 (38.8 arb.u) |
516.5 [8,10] |
|
3.1 Since CH, CH2 and C2 radicals are main species in CH4+H2 discharge plasma system with wire-plate electrodes at atmospheric pressure, the major resultants in the system are C2 hydrocarbons.
3.2 Since the work gas CH4 discharges are at atmospheric pressure and under high voltage and high frequency pulse conditions, the electric fields are provided with higher energy efficiency and higher degree of CH4 dissociation.
3.3 The optical emission spectrum online diagnoses determine that CH and CH2 species increase whereas C2 species decrease with the increase of supplied power. The results suggest that optimized CH4 discharge condition for resultants C2 hydrocarbons is about 60 W of supplied power with high voltage and high frequency pulse at atmospheric pressure.
3.4 Within the experiments, copper electrode sputtering is more violent with the increase of supplied power.
3.5 Black-body radiation produced by thermal movement of highly dense electrons is more violent with the increase of supplied power.
Acknowledgement We would like to take this opportunity to thank the State Key Laboratory of C1 Chemical Techniques of Chemical Engineering School of Tianjin University that provided us with some equipment for the study.
REFERENCES
[1] Fu C H, Yang Y J. Fundamentals of
Physics on Electronic Techniques. Guangzhou: Press of South China Institute of Technology,
1986, 195.
[2] Wang Y D, Wang Q J, Fu J S et al. Principles of Quantum Frequency Standard. Beijing:
Press of Science, 1986, 87.
[3] Krall N A, Trivelpiece A W. Principles of Plasma Physics (USA). Trans. Guo S Y, Huang
L, Qiu X M. Beijing: Press of Atomic Energy, 1983, 379
[4] Dagel D J, Mallouris C M, Doyle J R. J. Appl. Phys., 1996, 79 (11): 8735.
[5] Chen Z Z, Gao S X. Gas Electro-conduction (II). Nanjing: Press of Nanjing Institute of
Technology, 1988, 192.
[6] Inorganic Chemistry Teaching and Research Group of Beijing Normal University, Normal
College of Middle China and Normal College of Nanjing. Inorganic Chemistry, Beijing: Press
of Higher Education of China, 1981, 241.
[7] Wang H D, Ren L L, Gu J Q et al. Physical Experimentation ( Second Edition). Tianjin:
Press of Tianjin University, 1997, 486.
[8] Pearse R W B, Gaydon A G. The identification of molecular spectra (4th edition). New
York: Halsted Press, 1976, 83, 90.
[9] Qiu D R, Cheng W X. Spectrograms Scanned by Spectrograph with 2Å/mm & 4Å/mm
Gratings. Shanghai: Shanghai Press of Science and Technology, 1984, 29.
[10] Herzberg G, Spinks J W T. Molecular spectra and molecular structure (I) (2nd
edition). New York: Van Nostrand Reinhold Company, 1950, 31.