http://www.chemistrymag.org/cji/2004/066042ne.htm |
Jun. 1,
2004 Vol.6 No.6 P.42 Copyright |
Chang
Qing, Yu Mingquan, An Yu
(School of Environmental and Municipal Engineering, Lanzhou Jiaotong
University, Gansu 730070, China )
Abstract The removal of nickel ions and
turbidity from wastewater by Macromolecular Heavy Metal Flocculant is mainly discussed.
Compared with inorganic flocculants such as aluminium sulfate and polyaluminium
chloride, macromolecular Heavy Metal Flocculant are characterized by higher removal
efficiency, less dosage, lower value of the optimal pH, higher velocity of sedimentation.
When the pH of wastewater ranges from 5.5 to 6.0, the removal rates of nickel and
turbidity are 98% and above 98% respectively. The nickel ions and the substances causing
turbidity could prompt each other's removal in the treatment of wastewater containing both
nickel and the substances causing turbidity.
Keywords Macromolecular Heavy Metal Flocculant; dithioic acid; Nickel wastewater;
flocculation; wastewater treatment
Flocculation is one of the most important methods for wastewater treatment. In the flocculation process, the conditions necessary for chemistry and hydromechanics are given to colloidal suspension and then the particles become larger and separate from wastewater medium. Therefore the main objects of flocculation are the lyophobic colloids and suspended particles which consist of insoluble substances. It is nearly impracticable to remove the soluble substances directly by flocculant [1].However in some cases, the floc formed by flocculation could adsorb some soluble substances and precipitate together with them, this can be regarded as a cooperation effect. The soluble heavy metals ions in wastewater are permanent and not degradable pollutants. They can be adsorbed onto particles, or become particles by hydrolysis. Therefore the heavy metal content of wastewater can be reduced to some extent by flocculation. But there are still a quite number of heavy metals that are soluble in the polluted water. They are mainly metal complexing species with different ligands[2] and can not be effectively removed by present various flocculants.
For the above reason, a strong ligand of heavy metals (dithioic acid or salt group)[3-5] was introduced to a macromolecular flocculant (polyethyleneimine). The new kind of flocculant synthesized in this way has the function of trapping heavy metals and could remove not only the substances which cause the turbidity but also the various species of heavy metals from wastewater. This kind of flocculant is called Macromolecular Heavy Metal Flocculant (MHMF). Because MHMF has the two function, it is possible to reduce some treatment units in wastewater treatment, for example, chemical sedimentation, adsorption, ion exchange, membrane, so the wastewater treatment system could be simplified greatly. Also it must be pointed out that there has been no report on the research of MHMF up to now.
In this paper, the removal of nickel ions and turbidity from wastewater by MHMF is mainly discussed, and the performance of MHMF is evaluated compared with other inorganic flocculants.
2.EXPERIMENT
2.1Materials and instruments
(1) polyethyleneimine, MW10000
(2) carbon disulfide
(3) wastewater containing nickel ions;
(4) J6-1A Jar test instrument with 6 units
(5) 721Spectrophotometer
2.2 Methods of determination
(1) nickel ions: Diacetyldioxime Spectrophotometry
(2) turbidity: Spectrophotometry[6](standardized by diamine sulphate-urotropine)
2.3 Preparation of MHMF
The MHMF was prepared by the reaction of polyethyleneimine with carbon disulfide as follows:
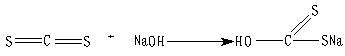 (1)
(1)
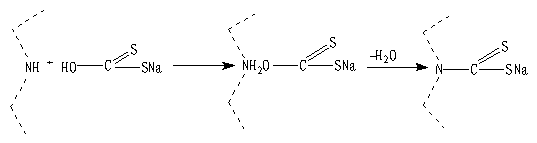 (2)
(2)
2.4 Jar test
The jar tests were conducted by J6-1A Jar test instrument with 6 units. First, the pH
values of the wastewater samples were adjusted to required values. After inputting MHMF,
the samples were stirred at a speed of 120 rpm for 2 minutes, followed by stirring at a
speed of 40 rpm for 10 minutes and sedimentation for 10 minutes[1]. The metal
concentration and turbidity of supernantant were assayed, when the above procedures of
test were finished.
3.RESULT AND DISCUSSION
3.1 The effect of pH value on removal rate of nickel or turbidity
The pH values of the wastewater samples containing only nickel (50mgL-1) or
only turbidity(100NTU) were adjusted to various values. Jar tests were done with these
samples. The dosages of MHMF are all the same (10mgL-1) in every test. The
results are shown in Fig.1:

Fig.1 The effect of pH value on the removal
rate
It is shown that the removal rate of nickel increases with the increase of pH value before the pH reaches 6.0. After pH reaches 6.0, the removal rate of nickel keeps constant. Also it is shown that the removal rate of turbidity is nearly 100% in the pH range of 3-5, but after the pH reaches 5.0, it quickly decreases with the increase of pH value.
3.2 The effect of MHMF dosage on removal rate
of nickel
The pH values of the wastewater samples containing nickel were adjusted to 5.5-6, and different dosages of MHMF were added into these samples in
jar tests. The original concentration of nickel in these samples are 25 mgL-1 (A),
50mgL-1 (B) and 100 mgL-1 (C), respectively .The results are shown
in Fig.2.
It is shown that there is an optimal dosage for every concentration of
nickel in sample£¬and the optimal dosages increase
with the increase of the concentration of nickel in sample. This shows the stoichiometric
relationships between the nickel and MHMF.
3.3 The effect of nickel and turbidity on each other's removal rate
3.3.1 The effect of turbidity on the removal rate of nickel
All the samples of wastewater contain the same concentration of nickel (50mgL-1), but
different turbidity (0NTU,100NTU,200NTU, respectively).
The pH values of these samples were adjusted to 5.5-6, and different dosages of MHMF
were added into these samples in jar tests. The results are shown in Fig3.
The experiment shows that the turbidity of wastewater can promote the
removal of nickel. The reason probably is that the floc formed from substances causing
turbidity has adsorption and sweep function [1] for soluble and insoluble nickel.

Fig.2 The relationships between the removal
of nickel and the dosages of MHMF

Fig.3 The effect of turbidity on the removal
of nickel with the dosages of MHMF
3.3.2 The effect of nickel on the
removal rate of turbidity
All the samples of wastewater have the same turbidity£¨100NTU,£©, but have different
concentration of nickel£¨0mgL-1 (A) ,
20mgL-1 (B) ,50mgL-1 (C) respectively£©. The pH values of these samples were adjusted to 5.5-6, and
different dosages of MHMF were added to the samples in jar tests. The results are shown in
Fig.4.
The experiment shows that the nickel in wastewater can promote the
removal of turbidity greatly. The reason probably is that the positive Ni2+
ions can neutralize the negative electric charge of dithioic radical group of MHMF
molecule because of the chelation, thus reducing the repulsion between MHMF and the
substances causing turbidity, therefore strengthening flocculation greatly.
3.4 Comparison between MHMF and traditional flocculants
The wastewater samples for jar test contain only nickel (50mgL-1), or only
turbidity (100NYU). After the pH values of these samples were adjusted to required values,
MHMF and the traditional flocculants, such as aluminium sulfate (AS) and polyaluminium
chloride (PAC), were added with the same concentration (50mgL-1) into these
wastewater samples respectively. The performances of different flocculants in the jar
tests were compared as shown in Fig.5.
It is shown that MHMF is much more efficient than PAC and AS for
removing nickel from wastewater in the all range of pH, and there is no need to raise pH
when using MHMF as the flocculant for general wastewater quality. But when using AS or PAC
as the flocculant, the pH of wastewater must be raised to a high value in order to raise
the removal rate of nickel. This is just about the traditional chemical sedimentation
method which has been used to remove the heavy metals in wastewaters up to now. It is
clear that the traditional chemical sedimentation method not only has much less effect
than flocculation by MHMF but also consume a large amount of basic material.
It is also shown that MHMF is much more efficient than PAC and AS for
removal of turbidity in the range of pH from 3 to 5. But when pH of wastewater is raised
to higher values, the removal rate of turbidity by MHMF decreases quickly with the
increasing of pH, and PAC becomes much more efficient than MHMF and AS.

Fig.4 The effect of nickel on the removal of turbidity

Fig.5 The comparision between MHMF and
traditional flocculants
Although the removal rate of turbidity by
MHMF decreases when pH of wastewater is raised in the range above 5.0, the nickel in
wastewater can promote the removal of turbidity greatly, as shown in Fig.4. So actually it
has no effect on the efficiency of treatment for the wastewater which contains both
turbidity and nickel.
3.5 Discussion of the mechanism
It is well known that polyethyleneimine is a kind of cationic flocculant because of the
different amino-groups in its macromolecule as follows:
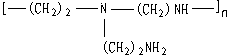
The nitrogen atoms must be protonated to achieve cationic charge, so the cationic charge density is pH dependent. Therefore the colloidal substances with negative charges can be flocculated by polyethyleneimine in the lower pH ranges because of charge neutralization, as the result, the turbidity of wastewater can be reduced. But the soluble heavy metal ions in wastewater can not be removed by it.
In this work, the dithioic acid (salt) groups are bound to the nitrogen atoms of polyethyleneimine. The new flocculant MHMF obtained by this way has the strong chelate effect for heavy metal ions and reacts with soluble heavy metals ions or soluble metal complexing species with other ligands producing insoluble chelate compound as follows:
 (4)
(4)For the above reason£¬MHMF not only has the function of flocculation but also has the function of trapping various species of heavy metal ions in wastewater.
Both the positive charge of polyethyleneimine main chain and the ionization equilibrium of the dithioic acid groups are influenced by the pH value of wastewater. When pH value of wastewater is raised to a higher value, the positive charges of the molecule decrease that the flocculation effect also decrease, but more dithioic acid groups ionize to acid radical that the chelate effect also increase. So there must be an optimal pH, at which the function of both flocculation and trapping heavy metal are ideal.
4.CONCLUSIONS
The new kind of flocculant MHMF has the function of trapping heavy metals and could
remove not only the substances which cause the turbidity but also various species of heavy
metals in wastewater.
MHMF is characterized by higher removal rate, less dosage, lower
optimal pH value, higher velocity of floc formation and sedimentation, compared with
inorganic flocculants, such as polyferric sulfate and aluminium sulfate.
Nickel ions and the substances causing turbidity have cooperative
removal effect with each other in the process of treating wastewater containing both
nickel and the substances causing turbidity.
REFERENCES
[1] Chang Q. The Flocculation Theory for Water Treatment (Shuichuli
Xuningxue). Beijing: China Chemical Industry Press, 2003 : 4-5, 50-53, 163-167.
[2] Dai S. Environmental Chemistry (Huanjing Huaxue). Beijing: China Higher Education
Press, 1997: 150-160.
[3] Zhang L, Qi W, Environmental Pollution & Control (Huanjing Wuran Yu Kongzhi),
1987, 9: 34-37.
[4] Gong S. Environmental Protection of Chemical Industry (Huagong Huanbao), 2001, 21:
95-97.
[5] Wing R E. Plating Surface Finishing, 1978, 65: 52-57.
[6] Chinese National Bureau for Environment Protection, The Methods of Monitoring and
Analysis for Water and Wastewater (Shui He Feishui Jiance Fenxi Fangfa) (4th
edition). Beijing: China Environmental Science Press., 2002: 375-377, 96-97.