http://www.chemistrymag.org/cji/2004/067051pe.htm |
Jul. 10,
2004 Vol.6 No.7 P.51 Copyright |
(Yunnan Academy of Tobacco Science, Kunming, Yunnan 650106; #School of Chemistry and Bioscience, Yunnan University of Nationality, Kunming, Yunnan 650031 )
Abstract A sensitive, selective
and rapid method for the determination of cobalt based on the rapid reaction of cobalt(II)
with 2-(2-quinolinylazo)-5-dimethylaminoaniline (QADMAA) and the solid phase extraction of
the Co(II)-QADMAA chelate with C18 membrane disks was developed. In the
presence of pH 5.5 buffer solution and cetyl trimethylammonium bromide (CTMAB) medium,
QADMAA reacts with cobalt to form a violet complex of a molar ratio 1:2 (cobalt to
QADMAA). This chelate was enriched by the solid phase extraction with C18
membrane disks. The enrichment factor of 50 was obtained by elution of the chelate form
the disks with minimal amount of isopentyl alcohol. In isopentyl alcohol medium, the molar
absorptivity of the chelate is 1.32×105
L.mol-1.cm-1 at 616 nm. Beer's law is obeyed in the range of
0.01- 0.6 mg/mL in the measured solution. The relative standard deviation for eleven
replicate sample of 0.01 mg/mL level is 1.35 %. The detection limit reaches 0.02 mg/L in the original samples.
This method was applied to the determination of cobalt in environmental samples with good
results.
Keywords 2-(2-quinolinylazo)-5-dimethylaminoaniline; cobalt, solid phase extraction
spectrophotometry
1 INTRODUCTION
Cobalt is an element both important for
industry and biological systems. In rapidly expanding the analytical fields such as
environmental, biological and material monitoring of trace metals, there is an increasing
need to develop simple, sensitive and selective analytical techniques that do not use
expensive or complicated test equipment. Many sensitive instruments, such as
spectrofluorimetry, X-ray fluorescence spectrometry, neutron activation analysis, atomic
absorption spectrometry, chemiluminescence, and the like have widely been applied to the
determination of cobalt.
In the previous works, some 2-quinolylazo-phenol reagents were reported for the determination of metal ions.[25-30] This kind of reagent has a higher sensitivity than pyridylazo reagents due to its larger conjugated system. However, the 2-quinolylazo-phenol reagent has also a disadvantage of its poor selectivity because both the oxygen atoms and nitrogen atoms donate elections to the metal ions. To select a more sensitive and selective reagent, in this work, a compound 2-(2-quinolinylazo)-5- dimethylaminoaniline (QADMAA) was synthesized, and its color reaction with cobalt was studied. This reagent has higher selectivity than that of 2-quinolylazo-phenol reagents because of it has only donating elections to metal ions.
The routine spectrophotometric methods are often not sensitive enough to determine low concentration of cobalt ion in environmental samples, as the cobalt concentration existing only at mg/L level. Consequently, a preconcentration step is usually required. Solid phase extraction is an attractive technique because of its notable advantages.[31-36] In this paper, based on the color reaction of QADMAA with cobalt and combining with the solid phase extraction of the colored chelate with C18 disks, a highly sensitive, selective and rapid method for the determination of cobalt in environmental samples was developed.
2 EXPERIMENTAL
2.1 Synthesis of QADMAA
2-Aminoquinoline (6.9 g) was dissolved in 500 mL anhydrous
ethanol. To which, sodamide (2.0 g) was added and the mixture was refluxed in a boiling
water bath for 5 h, followed by the addition of isoamyl nitrite (7.4 mL). The solution was
refluxed for 30 min with boiling water bath. The solution was cooled and placed over night
under 0°C. The diazo salt was
obtained by filtering this solution with an isolation yield of 95%. The diazo salt was
dissolved in 200 mL anhydrous ethanol, followed by the addition of m-dimethylaminoaniline
(5.7g; 0.042 mol). The carbon dioxide was ventilated into the solution with stirring until
the pH reaches to about 8.0. The solution was placed for two days, and evaporated to
dryness. The residue was re-crystallized with 30% ethanol. QADMAA was obtained with 36%
yield. The structure of QADMAA was verified by elemental analysis, IR (Fig.1), 1HNMR
(Fig.2), and MS (Fig.3). Elemental analysis: C17H17N5
found (calculated) C 69.82 (70.08), N 23.83 (24.04), H 6.04 (5.88). All these show that
the QADMAA has the structure in Fig.4.
Fig. 1 The infrared spectrum of QADMAA
Fig. 2 The 1H nuclear magnetic resonance spectrum of QADMAA
Fig. 3 The mass spectrum of QADMAA

Fig. 4 The structure of QADMAA
2.2 Experimental Apparatus
A UV-160A spectrophotometer (Shimidzu, Japanese) equipped with a 1 cm microcells
(0.5 ml) was used for all absorbance measurements. The pH values were determined with a
Beckman F-200
pH meter. The extraction was performed on Waters Solid Phase Extraction (SPE) device
(which can prepare twenty samples simultaneously), and Zorbax C18 membrane
disks [47 mm (diameter)× 0.5 mm (thickness), 8 mm, 50 mg] (Agilent Technologies, USA) was used.
2.3 Reagents
All of the solutions were prepared with ultra-pure water obtained from a
Milli-Q50 SP Reagent Water System (Millipore Corporation, USA). Isopentyl alcohol (Fisher
Corporation, USA) was used. QADMAA solution (5×10-4 mol/l) was prepared by
dissolving QADMAA with 95% of ethanol. A stock standard solution of cobalt (1.0 mg/mL) was
obtained from Chinese Standard Center, and a work solution of 1.0 m g/mL was prepared by
diluting this solution. The buffer solution of 0.5 mol.L-1 acetic acid-sodium
acetate (containing 5% of Zn-EGTA and 5% of NaF) was prepared by dissolving 30 g acetic
acid, 50 g sodium fluoride and 50 g glycoletherdiamine tetraacetic acid zinc salt
(Zn-EGTA) in 600 mL of water, then adjusted pH to 5.5 with sodium hydroxide solution and
diluted to volume of 1000 mL with water. Cetyl trimethylammonium bromide (CTMAB) solution:
1.0%, dissolving with 10% of ethanol. All chemical used were of analytical grade unless
otherwise stated.
2.4 General procedure
To a standard or sample solution containing no more than 2.4 m g of Co(II) in a 200 mL of
calibrated flask, 10 mL of 0.5 mol/L acetic acid-sodium acetate buffer solution
(containing 5% of Zn-EGTA and 5% of NaF) with pH 5.5, 6 mL of 5×10-4 mol/L
QADMAA solution and 5.0 mL of 1.0 % CTMAB solution were added. The mixture was diluted to
the volume of 200 mL and mixed well. After 10 min, the solution was passed through the C18
disks at a flow rate of 50 mL/min. After the enrichment had finished, the retained
chelates were eluted from the disks with 4 mL of isopentyl alcohol at a flow rate of 5
mL/min. The absorbance of the eluant was measured in a 1 cm cell at 616 nm against a
reagent blank prepared in a similar way without cobalt.
3 RESULTS AND DISCUSSION
3.1 Absorption Spectra
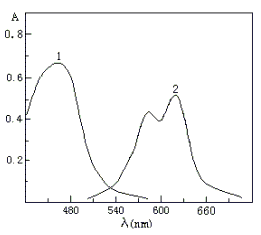
The absorption spectra of QADMAA and
its Co(II) complex in isopentyl alcohol medium are shown in Fig.5. The absorption peaks of
QADMAA and its complex are located at 462 nm and 616 nm.
 |
 |
|
| Fig.5 Absorption
spectra of QADMAA and its Co(II) complex: (1) QADMAA-CTMAB blank against water; (2) QADMAA-Co(II)-CTMAB complex against reagent blank |
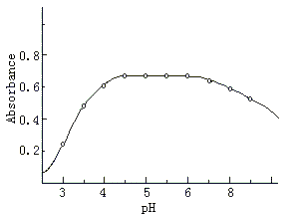
Fig.6 Effect of pH
on the formation of Co(II) complex, Co(II) concentration was 5.0×10-6 mol.l-1,
other conditions as general procedure. |
3.2 Effect of Acidity
Results showed that the optimal pH (Fig.6) for the
reaction of Co with QADMAA is 4.2- 6.8. A pH 5.5 acetic acid-sodium acetate buffer
solution was recommended to control pH. The use of 6-15 mL of buffer solution per 25 mL
was found to give a maximum and constant absorbance. So 10 mL of buffer solution was
recommended. In this work, the routine ions Ni(II), Cu(II), Zn(II) and Fe(III) react with
QADMAA to cause a serious positive interference. According to the literatures,
[37,38] for the determination of cobalt with pyridylazo reagent, the interference of
Fe(III) can be masked with NaF, and the interference Ni(II), Cu(II), Zn(II) can be masked
with Zn-EGTA. So the experiments of containing an appropriate amount of Zn-EGTA and NaF in
buffer solution to mask Ni(II), Cu(II), Zn(II) and Fe(III) ions were studied. The results
showed containing 4- 6% of Zn-EGTA and 3- 10 % of NaF in buffer solution can greatly
enhance the tolerance limits of Ni(II), Cu(II), Zn(II), Fe(III) and do not affect the
sensitivity of this method. So containing of 5% Zn-EGTA and 5% NaF in the buffer solution
were recommended to mask Ni(II), Cu(II), Zn(II) and Fe(III) ions.
3.3 Effect of surfactants
The Co-QADMAA complex has a poor solubility in water solution. It needs to add a
suitable amount of surfactant to enhance the solubility of the complex. Experiments showed
that all the anionic surfactants, nonionic surfactants and cationic surfactants have good
effect to enhance the solubility. In addition to enhance the solubility, in the nonionic
surfactants and cationic surfactants medium, the sensitivity of the Co-QADMAA chelates was
increased markedly too. The effect of the nonionic surfactants and cationic surfactants
for improving the sensitivity is shown in Table 1. The results showed that CTMAB was the
best additive and the use of 3.0- 8.0 mL of 10% CTMAB solution gave a constant and maximum
absorbance. Accordingly, 5.0 ml CTMAB solution was recommended.
Table 1 The effect of surfactants on Co-QADMAA chromogenic system
Surfactant |
Absence |
CTMAB |
CPB |
TritonX-100 |
Emulsifier-OP |
Tween-80 |
Tween-20 |
Tween-60 |
| lmax (nm) | 584 |
616 |
608 |
600 |
595 |
606 |
608 |
608 |
e (×104)l.mol-1.cm-1 |
8.01 |
13.2 |
10.2 |
8.85 |
9.17 |
10.4 |
8.56 |
8.68 |
3.4 Effect of QADMAA
concentration
For up to 2.4 mg of Co(II), the use of about 5 -10 mL of 5×10-4 mol/L of QADMAA
solution has been found to be sufficient for a complete reaction. Accordingly, 6 mL of
QADMAA solution was added in all further measurement.
3.5 Stability of the chromogenic system
After mixing the components, the absorbance reaches its maximum within 6 min at room
temperature and remains stable for 6 h in aqueous solution. The chelates are stable at
least 20 h when it was extracted into the isopentyl alcohol medium.
3.6 Solid phase extraction
Both the enrichment and the elution were carried out on a Waters SPE device (which can
prepare twenty samples simultaneously). The flow rate was set to 50 mL/min during
enrichment and 5 mL/min while elution.
Some experiments were carried out in order to investigate the retention
of QADMAA and its Co(II) chelate on the disks. It was found that the QADMAA and its Ni(II)
chelate was retained on the disks quantitatively when they pass the disks as aqueous
solution. The capacity of the disks for QADMAA was 31 mg and for its Co(II)-chelate was 22
mg in a 200 mL of solution. In this experiment, the disks has adequate capacity to enrich
the Co(II)-QADMAA chelate and the excessive QADMAA.
In order to choose a proper eluant for the retained QADMAA and its
Co(II) chelate. Various organic solvents were studied. The effect of various organic
solvents was in the following sequence: isopentyl alcohol > acetone > acetonitrile
> ethanol > methanol. So isopentyl alcohol was selected as eluant. Experiment show
that it was easier to elute the retained QAMAA and its Co(II) chelate in reverse direction
than in forward direction, so it is necessary to upturned disks when elution. 4.0 mL of
isopentyl alcohol was sufficient to elute the QADMAA and its Co(II) chelate from disks at
a flow rate of 5 mL/min. The volume of 4.0 mL eluant was selected.
3.7 Calibration Curve and Sensitivity
The calibration curve show that Beer's law is obeyed in the concentration range of
0.01~ 0.6 m g Co(II) per mL, The linear regression equation obtained was: A = 2.28 C
(mg/mL)
+ 0.0115, (r=0.9991). The molar absorptivity was calculated to be 1.32×105L.mol-1.cm-1
at 596 nm. The relative standard deviation at a concentration level of 0.2 mg Co(II) per mL (11
repeat determination) was 1.35 %.
3.8 Composition of the complex
The composition of the complex was determined by continuous variation and molar ratio
method. Both showed that the molar ratio of Co(II) to QADMAA is 1:2.
3.9 Interference
The selectivity of the proposed method investigated by the determination of 2.0 mg/200ml of Co(II) in
the presence of various ions within a relative error of ± 5% are given in Table 2.
Ion added |
Tolerate (mg) |
tartrate, thiourea, NO3-, Cl- , Na+, K+, borate, |
50 |
oxalate, citrate, benzoate, succinate, ascorbate, SO42-, NH4+ |
20 |
Li+, Al3+, PO43- |
10 |
F-, Br-, ClO4- , I- , Ca2+, Mg2+ |
5 |
Sr2+, Ce(IV) , Ba2+, Zr(IV) , Zn2+, Fe3+ |
2 |
Mn2+, W(VI), Mo(VI) , Cu2+ |
1 |
Ti(IV), Bi(III), V(V), Cr(VI), Zr(IV), Ni2+, Th(IV) |
0.5 |
Tl(III), Ag+, Cd2+, Cr3+, Fe2+, La3+, Sn(IV) , Pb2+ |
0.2 |
Ru(III), Bi(III), Hg 2+, Sb3+ , Pd2+, Sn(IV) , Sb(III) |
0.1 |
Se(IV), U(VI), Te(IV), Au3+, Pt2+ |
0.03 |
3.10 Application
The proposed method has been successfully applied to
the determination of cobalt in biological sample and water.
For biological samples, 0.20 g of sample was weigh accurately and put
into the Teflon high-pressure microwave acid-digestion bomb (Fei Yue Analytical Instrument
Factory, Shanghai, China). 2.5 mL of concentrated nitric acid and 2.5 mL of 30% hydrogen
peroxide were added. The bombs were sealed tightly and then positioned in the carousel of
the microwave oven (Model WL 5001, 1000 W, Fei Yue Analytical Instrument Factory,
Shanghai, China). The system was operated at full power for 6.0 min. The digest was
evaporated to near dryness. The residue was dissolved with 1% of hydrochloric acid, and
the cobalt contents were analyzed according to general procedure. The results are shown in
Table 3.
For water sample, the samples were acidified with hydrochloric acid and
filtrated by 0.45 mm filter. The cobalt contents were analyzed according to general procedure. The
results were shown in Table 4, together with the results of a recovery test. A standard
method using ICP-MS has also been used as reference method. The results are also shown in
Table 4.
Table 3 Determination of cobalt in the certified standard biological samples
Samples |
Standard value ( mg/g) |
By this method |
RSD% |
Human hair (GBW07601) |
As(0.28), B(1.3), Bi(0.34), Ca(2900), Cd(0.11), Ce(1.2), Co(0.71), Cr(0.37), Cu(10.2), Fe(54), Hg(0.36), Mg(360), Mn(6.3), Mo(0.073), Ni(0.83), Pb(8.8) |
0.715 |
2.8 |
Tea Leaf (GBW08505) |
As(0.191), Ba(15.7), Ca(2840), Cd(0.032), Co(2.2), Cr(0.8), Cu(16.2), Fe(373), Hg(0.004), Mg(2240), Mn(766), Ni(7.61), Pb(1.06), Se(0.041), Zn(38.7), |
2.31 |
2.5 |
Samples |
Reference method ( mg/L) |
Found * |
RSD% |
Recovery (%) (n=5) |
River water |
61.7 |
63.4 |
2.4 |
93 |
Lake water |
45.8 |
46.2 |
2.8 |
105 |
Tap water |
34.5 |
35.6 |
2.5 |
97 |
Table 5 Comparison of reagents for spectrophotometric determination of cobalt
Reagent |
Medium/solvent |
lmax (nm) |
e (×104) |
Linear range ( mg.ml-1) |
Ref |
1-(2-Pyridylazo)-2-naphthol |
Aqueous (Triton X-100) |
620 |
1.9 |
0.4-3.2 |
8 |
4-(2-Pyridylazo)-resorcinol |
CHCl3 |
520 |
6.4 |
- |
9 |
4-(2-Thiazolyazo)- resorcinol |
Aqueous |
510 |
6.0 |
0.2-2 |
12 |
2-(2-Benzothiazolyazo)-2-p-cresol |
Aqueous (Triton X-100) |
615 |
1.62 |
0.08-1.06 |
14 |
Sodium diethyldithiocarbamate |
CCl4 |
322 |
2.33 |
- |
15 |
1-Nitroso-2-naphthol |
Aqueous (Triton X-100) |
420 |
3.18 |
0.2-3 |
16 |
2-Hydroxybenzaldehyde-5-nitro-pyridylhydrazone |
Aqueous(CTMAB), Separation by IRC-718 resin |
470 |
6.5 |
0.02-2 |
17 |
a -Benzilmonoxime |
CHCl3 |
380 |
2.55 |
0.08-2.2 |
18 |
5-[o-Carboxyphenylazo]-2,4-dihydroxybenzoic acid |
Aqueous (Tween-80) |
550 |
1.64 |
0.1-3.5 |
19 |
5-(4-Sulfophenylazo)-8-aminoquinoline |
Aqueous (CTMAB) |
560 |
6.42 |
0.1-2 |
20 |
Nitroso-R Salt |
CHCl3 |
420 |
3.30 |
0.1-2 |
21 |
2-(3,5-Dibromo-2-pyridylazo)-5-dimethylaminobenzoic acid |
CH2Cl2 |
673 |
15.5 |
0.0-0.35 |
22 |
2-(3,5-Dibromo-2-pyridylazo)-diaminotoluene |
pH 4-8 |
590 |
13.4 |
0.01-0.4 |
23 |
5,10,15,20-Tetrakis-(4-sulfophenyl)-porphine |
pH 4.0 |
426 |
35.4 |
0.0-0.2 |
24 |
2-(2-Quinolylazo)-5-dimethyl aminoaniline |
Aqueous (CTMAB) |
616 |
13.2 |
0.01-0.6 |
This work |
A comparison of the possibilities of the QADMAA with those of other methods for the determination of cobalt was given in Table 5. In this work, a high sensitive and selective reagent for cobalt was synthesized. Experiments show that the molar absorptivity of the Co(II)-QADMAA-CTMAB chelate reaches 1.32×105 L.mol-1.cm-1 in the measured solution. Most foreign ions do not interfered with the determination in the presence of sodium thiosulfate and sodium fluoride medium. By solid phase extraction with C18 disks, the enrichment factor of 50 was achieved. The detection limit reaches 0.02 mg/L in the original samples, and low concentration of cobalt in samples can be determined with good results. The consuming of organic solvents in this method is much lower than those consumed in liquid-liquid extraction method. By using Waters SPE device, twenty samples can be prepared simultaneously. This method is rapid for simultaneously preparing large amount of samples.
Acknowledgement The project was granted by Yunnan China Tobacco Industry Corporation (2001B204).
REFERENCES
[2] Yuzefovsky A I, Lonardo R F, Michel R G, Anal Chem., 1995, 67: 2246-2253.
[3] Chow A, Yamashita G F, Hamon R F. Talanta, 1981, 28: 437-445.
[4] Cao Q E, Zhao Y K, Wu S Q et al. Talanta, 2000, 51: 615-623.
[5] Maziere B, Gros J, Comar D J. Radioanal Chem, 1975, 24: 707-714.
[6] Ma W J, Guo M D. Chinese Journal of Physical and Chemical Analysis, Chemical analysis part (Lihua Jianyan Huaxue Fence), 1999, 35: 284-286.
[7] Li S Q, Wang L P, Zhou C Y. Chinese journal of Netallurgical Analysis (Yejin Fenxi), 2000 20: 34-37.
[8] Watanabe H. Talanta, 1974, 21: 295-301.
[9] Siroki L M, Maric L, Stefanac Z et al. Anal.Chim. Acta, 1975, 75: 101-107.
[10] Khan M R, Khoo S B. Analyst, 1998, 123: 1351-1358.
[11] Ferrira S L C, De Jesus D S. J Braz Chem, 1996, Soc 7: 109-110.
[12] Busev A I, Nemtseva Z I, Ivanov V M. Zh Anal Khim, 1969, 24: 1376-1380.
[13] Shen H X, Li J J. Chemical Journal of Chinese Universities (Gaodeng Xuexiao Huaxue Xuebao), 1988, 9: 1007-1009.
[14] Carvalho M S, Fraga I C S, Neto K C L et al. Talanta, 1996, 43: 1675-1680.
[15] San Andres M P, Marina N L, Vera S. Talanta, 1994, 41: 179-186.
[16] Singh H B, Agnihotri N K, Singh V K. Talanta, 1999, 48: 623-631.
[17] Park C I, Cha K W. Talanta, 1998, 46: 1515-1523.
[18] Eskandari H, Ghaziaskar H S, Ensafi A A. Anal Sci, 2001, 17: 327-331.
[19] Amin A S, Ahmed I S, Moustafa M E. Anal Let, 2001, 34: 749-759.
[20] Zhao J W, Xu Q H. Chinese journal of chemical reagent (Huaoxue Shiji), 1992, 14 (6): 346.
[21] National Standards of P.R.China, GB/T 8638.7-88
[22] Katami T, Hayakawa T. Analyst, 1983, 108: 864-869.
[23] Shibata S, Furukawa M, Kamata E. Anal Chim Acta, 1974, 73: 107-119.
[24] Ni Q D, Zhang Y H. Chinese journal of analysis laboratory (Fenxi Shiyanshi), 1994, 13 (6): 56-57.
[25] Gusev S I, Nikolaeve E M, Pirozhkova E A. Zh Anal Khim, 1975, 30: 3-8.
[26] Ishizuki T, Tsuzkui M, Yuchi A et al. Anal Chim Acta, 1993, 117: 161-167.
[27] Simgh I, Poonam M. Talanta, 1984, 31 (2): 109-112.
[28] Barua S, Varma Y S, Garg B S et al. Analyst, 1981, 105: 996-998.
[29] Chen J H, Hu Q F, Yin J Y. Chinese journal of Spectroscopy Laboratory (Guangpu Shiyanshi). 2001, 18 (3): 341-343.
[30] Hu Q F, Yang G Y, Tang D Y et al. Chinese journal of arid environmental monitoring (Ganhan Huanjing Jiance), 2001, 15 (4): 297-299.
[31] Yang G Y, Zhang C M, Hu Q F et al. J.Chromatog.Sci, 2003, 41 (4): 195.
[32] Garg B S, Sharma R K, Bhojak N et al. Microchem. J., 1999, 61: 94.
[33] Pyrzynska K, Trojanowicz M. Critical reviews in Analytical Chemistry, 1999, 29, 313.
[34] Hu Q F, Yang G Y, Yin J Y. Talanta, 2002, 57, 751-756.
[35] Keil O, Joachim D, Dietrich A V. J.Chromatogr. Sci, 1997, 35, 519.
[36] Haddad P R, Doble P, Macka M. J Chromatogr. A, 1999, 856, 145.
[37] National Standards of P.R.China, GB 223.21-85
[38] Zhang G X, Zheng D Q. Speparation procedures in inorganic Analysis. Shanghia Science & technology Press, Shanghia China, 1989, 163-270.
固相萃取分光光度法测定环境样品中的钴
翁哲慧, 贾智若, 董学畅# , 缪明明 胡秋芬#
(云南烟草科学研究院, 昆明, 云南 650106; #云南民族大学化学与生物技术学院, 昆明, 云南 650031 )
摘要 基于钴(II)和2-(2-喹啉偶氮)-5-二甲氨基苯氨(QADMAA)的快速反应以及C18固相萃取盘对Co(II)-QADMAA螯合物的固相萃取,建立一种灵敏度高、选择性好、快速的钴测定方法。在pH=5.0的缓冲液和十六烷基三甲铵溴化胺(CTMAB)介质中,QADMAA和钴形成络合比为2:1的紫红色络合物。将此络合物用C18固相萃取盘进行固相萃取,用少量的异戊醇可洗脱,富集倍数为50倍。在异戊醇介质中,络合物在616 nm处的摩尔吸光系数为1.32×105 L.mol-1.cm-1。钴含量在0.01- 0.6 mg/ml范围内符合比尔定律。对0.01 mg/ml的样品11次重复测定,相对标准偏差为1.35%。样品中的检测限达到0.02 mg/L。将此方法用于环境样品中钴的测定,结果令人满意。
关键词 2-(2-喹啉偶氮)-5-二甲氨基苯氨,钴,固相萃取光度法