http://www.chemistrymag.org/cji/2004/06a071pe.htm |
Oct. 1,
2004 Vol.6 No.10 P.71 Copyright |
The effects of
F- on the synthesis of zeolite ZSM-35 by a vapor phase transport method Li Xuewu, Wei Zhijuan, Xu Hong, Dong Jinxiang(Research Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China)
Received June 28, 2004; Supported by the National Natural Science Foundation of China (No. 20373047)
Abstract In the system of NH4F-Al2O3-SiO2-ethylenediamine (EDA)-H2O, zeolite ZSM-35 was synthesized by a vapor phase transport method and the effects of F- were investigated in the synthesis of zeolite ZSM-35. Typical sample using NH4F as mineralizer possessed the higher crystallinity, larger particle ( up to 60mm) and higher surface area.Keywords ZSM-35, vapor phase transport method, mineralizer, crystallinity.
1. INTRODUCTION
The vapour phase transport method
[1] is a method of synthesis for zeolites. It could decrease the
consumption of organic materials. In addition, the existence of solid phase made this
method have the opportunity of carrying out in situ synthesis and controlling the chemical
components of zeolites[2]. It was used for synthesizing many zeolites and
molecular sieves, such as zeolite MCM-22[3], zeolite MFI[4], zeolite
MOR[5], molecular sieve AlPO4-5 and AlPO4-11[6],
molecular sieve VPI-7[7], and so on.
In the system of
hydrothermal zeolite synthesis, the replacement of the hydroxide anions by fluoride anions
as mineralizers made it possible to obtain zeolite even in slight acidic media. As its
application extended from high pH systems to low pH systems, novel silica polymorph [8],
large single crystal[9](molecular sieve AlPO4-5) and ultrastable
zeolite ITQ-21[10] could be synthesized.
So far, the effects of fluoride ion on zeolites
synthesis were not investigated in vapor phase system. Here, we report the result that
ZSM-35 was synthesized using NH4F instead of NaOH in order to investigate the
effects of fluoride ion in the system of zeolite synthesis by the vapor phase method. XRD,
SEM, FT-IR, nitrogen adsorption were used to characterize the typical synthesized samples.
2. EXPERIMENTAL
2.1 Synthesis
Distilled water and amorphous aluminosilicate gel[1] were prepared by ourself;
fluoride salt ( NH4F, min. 96.0 wt.%) , sodium hydroxide (min. 96.0 wt.%) and
EDA (min. 99.0 wt.%) are chemical reagents.
Distilled water was initially introduced into crucible; secondly, NH4F
or NaOH was added to distilled water under stirring condition; finally, aluminosilicate
gel was added to the crucible in the same
way. Beforehand, the reaction
liquid mixture containing distilled water and EDA was poured into an autoclave. The prepared aluminosilicate gel was put
into the container with sieves at its bottom. The container was placed on the supporter of the
autoclave. The autoclave was sealed and left to stand in a dry oven. Reaction was carried
out at 493 K for 15 days. After the crystallization of reaction mass was over, the solid
samples were washed with distilled water and dried at 373 K.
2.2 Characterization
Powder X-ray diffraction ( XRD) data were collected on a Rigaku D/max 2500 diffractometer
using CuK¦Á radiation.
The percentage crystallinity was calculated by comparing the sums of intensities of the
peaks appearing at 9.4¡ã, 22.4¡ã, 22.7¡ã, 23.3¡ã, 23.7¡ã, 24.5¡ã, 25.3¡ã and 25.8¡ãin the
synthesized samples to those same intensities found in the fully crystalline reference
material. The morphology and size of the zeolite ZSM-35 were determined using a JEOL
JSM-35C scanning electron microscopy ( SEM) . Furthermore, the framework vibrations of the
zeolites were determined using a Perkin-Elmer-1700 infrared spectroscopy spectrometer from
KBr pellets. Nitrogen adsorption measurements were performed to estimate Langmuir surface
area of typical samples.
3. RESULTS AND DISCUSSION
3.1 The effect of the Si/Al ( molar ratio)
The initial molar composition of the solid reaction gel was: NH4F/Al2O3=40.0,
H2O/Al2O3=57.4. The molar composition of the liquid
reaction mixtures was: EDA/H2O=0.8. Crystallization time and temperature were
15 days and 493 K, respectively. The effects of the Si/Al ratio on synthesizing zeolite
ZSM-35 were studied. Zeolite ZSM-35 could be obtained in relatively wide range of SiO2/Al2O3
ratio, 13.6~ 41.1. Meanwhile, the degree of crystallinity of zeolite ZSM-35 decreased with
increasing SiO2/Al2O3 ratio ( see Table.1) . However, for
SiO2/Al2O3=47.7, synthesized zeolites were both zeolite
ZSM-35 and ZSM-5, that is to say, the product was the mixture of zeolite ZSM-35 and ZSM-5.
Table.1 The effect of SiO2/Al2O3 ratios on the synthesis of ZSM-35
Sample |
SiO2/Al2O3 |
Crystallinity of zeolite ZSM-35( % ) |
Other phase |
1 |
13.6 |
100 |
- |
2 |
20.2 |
98.5 |
- |
3 |
24.5 |
96.3 |
- |
4 |
41.1 |
48.8 |
- |
5 |
47.7 |
40.6 |
ZSM-5 |
The gel composition was kept at constant SiO2/Al2O3=13.6, H2O/Al2O3=57.4 and NH4F/Al2O3=40. Crystallization time and crystallization temperature were 15 days and 493 K, respectively. The effects of EDA/H2O ratio on synthesis of zeolite ZSM-35 were studied (see Table 2). The data appeared that only a proper range of EDA/H2O ratio (0.3~ 0.8) resulted in pure crystal phase of zeolite ZSM-35 and its crystallinity increased with increasing EDA/H2O ratio. The sample was the mixture of zeolite ZSM-35 and ZSM-5 when EDA/H2O ratio was up to 1.0.
Table 2. The effect of EDA/H2O ratios on the synthesis of zeolite ZSM-35
Sample |
EDA/H2O |
Crystallinity of zeolite ZSM-35( % ) | Other phase |
1 |
0 |
0 |
Amorphous |
2 |
0.2 |
0 |
Amorphous |
3 |
0.3 |
88.9 |
- |
4 |
0.5 |
93.9 |
- |
5 |
0.7 |
95.6 |
- |
6 |
0.8 |
100 |
- |
7 |
1.0 |
82.6 |
ZSM-5 |
3.3 The effect of fluoride salt on
the synthesis of zeolite ZSM-35
The aluminosilicate gel with SiO2/Al2O3=13.6, H2O/
Al2O3=57.4 was used in the system of ZSM-35 synthesis.
Crystallization time and temperature were 15 days and 493 K, respectively. The effects of
fluoride salt and alkaline ion were systematically investigated under the vapor of EDA and
H2O. The results were showed in Table 3 and 4.
Table.3 The effect of NH4F/Al2O3 ratios on the synthesis of zeolite ZSM-35
Sample |
NH4F/Al2O3 |
Crystallinity of zeolite ZSM-35( % ) ) |
1 |
0 |
0 |
2 |
5 |
32.3 |
3 |
15 |
93.1 |
4 |
20 |
96.7 |
5 |
25 |
99.0 |
6 |
40 |
100 |
Table.4 The effects of NaOH/ Al2O3 ratios on the synthesis of zeolite ZSM-35
Sample |
NaOH/Al2O3 |
Crystallinity of zeolite ZSM-35( % ) |
1 |
1.0 |
51.3 |
2 |
2.0 |
55.2 |
3 |
3.0 |
57.3 |
4 |
4.0 |
63.5 |
From the above results it
could be seen that pure zeolite ZSM-35 was obtained using synthesis mixtures with NH4F/Al2O3=5-
40 and NaOH/Al2O3=1.0- 4.0, respectively. The degree of
crystallinity of zeolite ZSM-35 using NH4F as mineralizing agent was higher.
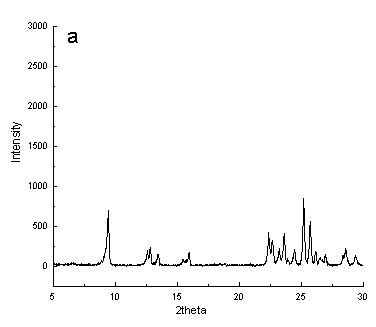
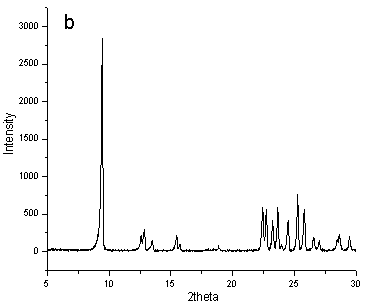
Fig.1 XRD patterns of typical samples synthesized ( a) using NaOH, ( b)
using NH4F
3.4 Properties Characterization
The typical samples ( Sample 6 in Table 3 and Sample 4 in Table 4) were chosen to
investigate their physical and chemical properties, systematically.
Theirs X-ray diffraction patterns were given in Figure 1a and b.
Comparing with the literature [11], their positions and relative intensity of
diffraction peaks using different inorganic materials as mineralizing agent were similar
to zeolite ZSM-35. The data proved the synthesized samples were pure phase of zeolite
ZSM-35. At the same time, their percent crystallinities were different. According to
Figure 1, apparently, the degree of crystallinity of synthesized sample using NH4F
as mineralizing agent was higher.

Fig.2 SEM pictures of ( a) synthesis typical sample using NaOH, ( b)
synthesis typical sample using NH4F.
The morphology of
synthesized zeolite ZSM-35 was shown in Figure 2. According to their SEM photos, synthesis
zeolite ZSM-35 using NH4F as mineralizing agent appeared in the form of
irregular flake with a length of 60¦Ìm ( Figure 2b) ;
however, the length of zeolite ZSM-35 using NaOH as mineralizing agent is about 10¦Ìm ( Figure 2a) . Apparently, fluoride ion advantaged the synthesis
of large crystals. The role of fluoride ion using vapor phase transport method was similar
to that of fluoride ion in hydrothermal zeolite synthesis.

Fig.3 IR spectra of various typical samples. ( a) using NaOH, ( b) using
NH4F
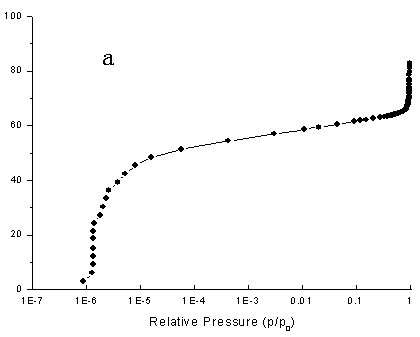
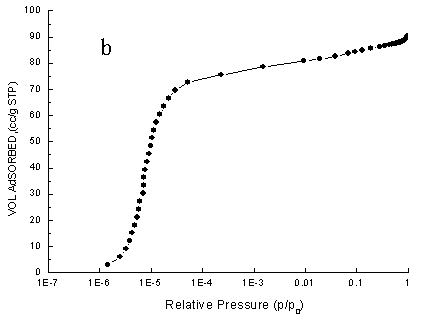
Fig.4 Nitrogen adsorption isotherms of typical samples ( a) using NaOH, (
b) using NH4F
IR spectra of the
typical samples were given in Figure 3. According to the IR spectroscopy patterns of the two typical
samples ( see Figure 3) , the sites of their main peaks were almost the same.
Meanwhile, according to the IR spectroscopy patterns of the literature [12] the
two typical samples were zeolite ZSM-35.However, the Fig.3b possessed an adsorption peak
at 670 cm-1 assigned to symmetrical stretch vibrations of internal tetrahedral
because of the effects of fluoride anion while the 3a did not. In addition, the framework
Si/Al ratio of synthesis sample using NH4F as mineralizing agent was higher
according to the principle[13] that Al content increases with framework
vibration frequency shifting toward the low frequency bands.
The nitrogen adsorption isotherms
of the zeolite ZSM-35 samples were presented in Figs.4a and b. The two isotherms were very
similar according to Fig.4. Certainly, their adsorption isotherms belonged to Langmuir
type adsorption. Meanwhile, Langmuir surface area of synthesized zeolite ZSM-35 using NH4F
as materializing agent was 373 m2/g. However, Langmuir surface area of
synthesized zeolite ZSM-35 using NaOH as materializing agent was 273 m2/g. The
above data showed synthesized zeolite ZSM-35 using NH4F as materializing agent
had high crystallinity.
4. CONCLUSION
By employed the vapour phase transport method, zeolite ZSM-35 can be synthesized in
the system NH4F-Al2O3-SiO2-ethylenediamine-H2O.
The amorphous gel composition should be ( 5- 40) NH4F: ( 13.5- 41.1) SiO2:
Al2O3: 57.4H2O for getting zeolite ZSM-35. The
experimental data proved that F- had the function of increasing crystalline
size and improving relative crystallinity. The effects of F- in the vapor phase
transport were similar to that of F- in the hydrothermal system.
[1] Xu W Y, Dong J X, Li J P et al. J. Chem. Soc., Chem. Commun., 1990, 755.
[2] Andreas A, Stefan S, Michael H et al. Microporous and Mesoporous Materials, 2003, 62 (1-3) : 97.
[3] S Inagaki, M Hoshino, E Kikuchi et al. Studies in Surface Science and Catalysis, 2002, 142: 53.
[4] Dong J X, Liu G H, Li J Q. Acta Petrollei Sinica (Petroleum Processing Section) (Shi You Xue Bao (Shi You Jia Gong)), 1995, 11 (3) : 63.
[5] Dong J X, Dong P, Liu G H et al. Journal of Fuel Chemistry And Technology (Ran Liao Hua Xue Bao), 1997, 25 (1) : 7.
[6] Li J P, Liu G H, Cao J H et al. Acta Petrollei Sinica (Petroleum Processing Section) (Shi You Xue Bao (Shi You Jia Gong)), 1997, 13 (1) : 36.
[7] Dong J X, Xue C F, Liu G H. Studies in Surface Science and Catalysis, 2002, 142: 431.
[8] Flanigen E M, Patton R L. U. S. Patent, 4,073,865, 1978.
[9] Qiu S L, Pang W Q, Henry Kessler et al. Zeolites, 1989, 9 (5) : 440.
[10] Avelino Corma, Maria J. Diaz-Cabanas, Joaquin Martinez-Triguuero et al. Letters to Nature, 2002, 418 (1) : 514.
[11] Xie S J, Wang Q X, Xu L Y et al. Chinese Journal of Catalysis, 2000, 21 (4) : 297.
[12] Yuan Z Y, Zhang H B, Wang J Z et al. Chinese Chemical Letters, 1997, 8 (9) : 836.
[13] Team of Molecular Sieve, Da Lian Institute of Chemical Physics, The Chinese Academy of Sciences. Zelites Molecular Sieve, Beijing: Science Publishing House, 1978: 171. ¡¡