http://www.chemistrymag.org/cji/2004/06a072pe.htm |
Oct. 1,
2004 Vol.6 No.10 P.72 Copyright |
The electrochemical study of
w-mercaptohexanoic acid self-assembled monolayer modified gold electrodeTu Yifeng
, Sun Ru, Gu Renao
(College of Chemistry and Chemical Engineering, Suzhou University, Suzhou, 215006, China)
Received July 21, 2004.
Abstract
An w-mercaptohexanoic acid self-assembled monolayer modified gold electrode was studied in this paper. The surface structure and properties were characterized by surface enhanced Raman scattering spectrum and electrochemical method. The experimental results showed that the double-layer capacitance of electrode decreased from 71m F/Cm2 to 18m F/Cm2 after forming a high coverage(96%) self-assembled monolayer. The electrons transfer associated with the Fe(CN)63- /Fe(CN)64- redox reaction is considered as an electron tunneling process, the apparent electron transfer rate constant is 2.47¡Á 10-5 cm/s.Keywords w-Mercaptohexanoic acid; Self-assembled monolayer; Chemical modification; Electrochemical property
1. INTRODUCTION
The self-assembled monolayers(SAMs)[1] have the remarkable characteristics
of in situ spontaneous formation, thermodynamical steady, highly ordered, densely stacking
and low defect. The sulfhydryl alkane compounds is a kind of priority species in this
field due to their peculiar molecular structure[2] and the reaction with Au[3].
The bond energy of Au-S (184KJ/mol) is strong enough to ensure the selectivity of the
adsorbing process. The characters of outer surface and the order degree of SAMs are mainly
influenced by the length of alkyl chain and the property of end group -R[4].
The ordered monolayer is formed if the carbon number is larger than nine. And the
influence on the Au/S interface will be shielded by the carbon chain when it was longer
than five. These are the important reasons of why the w-mercaptohexanoic acid(6-MHA) had been selected to carry on the
researches in this paper.
2. EXPERIMENTS
2.1 Instruments and chemicals
A RDE4 Potentiostat (Pine Corporation, USA) and a BAS-100A Electrochemical
Analyzer (Bioanalytical Systems Inc., USA) were used in all of electrochemical studies. A
HRD-1 Raman Spectrometer (Jobin Yvon Corporation, France) with a 40mW 488.0nm Argon Laser
(A-240 Type) was used to characterize the surface properties.
w-Mercaptohexanoic
acid[5] was synthesized in our laboratory. It had been identified by FT-IR and
NMR. IR Wavenumbers(cm-1): 733.0(C-S), 1709.1(C=O), 2573.2(S-H), 2858.7,
2935.9(both CH2 stretch), dH of 1H-NMR(CDCl3) (ppm): 1.328(t,
SH), 10.850(s, COOH).
Acetone, potassium ferricyanide, sulfuric acid and other reagents are
all of analytical grade.
2.2 The preparation and modification of electrode
The surface of a gold electrode was polished and then washed with acetone and redistilled
water in an ultrasonator. In 1.0mol/L H2SO4 solution, with a SCE
reference electrode and a Pt auxiliary electrode, cyclic scan in range of -0.2V to1.2V to
pretreat the electrode surface till a steady voltammogram was obtained. Then, cyclic scan
in 0.1mol/L KCl solution with scanning rate of 500mV/S to obtain a rough electrode surface[6].
The true surface area equals to 0.042cm2 which was calculated from the result
of coulometric electrolysis[7]. After washed by redistilled water and acetone,
the pretreated electrode was dipped into 0.1mol/L 6-MHA solution of acetone under the
protection of pure nitrogen gas to bring about the self-assembling modification.
2.3 Study of the electrochemical properties of 6-MHA SAM
Cyclic voltammetric scanned from -0.2V to 0.8V (vs. SCE) for studying the double layer
capacitance of electrodes, and use the Fe(CN)63-/4- as the probe to
study the transfer process of the electrons by CV.
3. RESULTS AND DISCUSSION
3.1 Characterization of the SAM by Surface Enhanced Raman Scattering
Spectrum(SERS)
It was proved by SERS(see Fig. 1) that the 6-MHA molecules were linked on the surface of
gold electrode. The stretching vibration bands of S-H of 6-MHA(2574 cm-1 on
curve a) disappeared on the spectrum of 6-MHA SAM, and the C-S stretching vibration
band(714cm-1 on curve a, 807 cm-1 on curve b) was enhanced. It could
be concluded that the S-H bond in 6-MHA had been replaced by S-Au bond due to the strong
affinity between S and Au. The other atoms of the carbon chain ranged away from electrode
surface gradually, so the C-S stretching vibration was enhanced. The 6-MHA molecules
linked on the surface of gold electrode by C-S bond, the -COOH groups were far apart from
the surface.
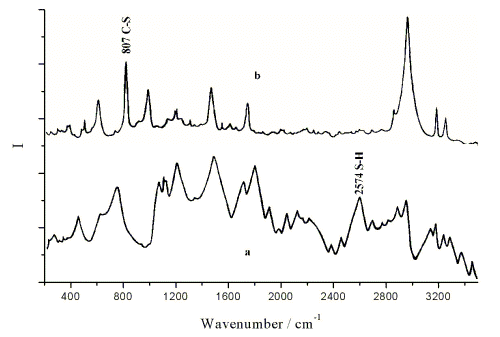
Fig.1 (a) Raman spectrum of 6-MHA in liquid
and (b) SERS spectrum of 6-MHA SAM on gold electrode
3.2 The change of double layer capacitance
The experimental results had proved that, when the exterior orientational arranged water
molecules of electrode were replaced by organic molecules, the double layer capacitance
would decrease on account of the larger distance between the charged layers and the
smaller dielectric constant. The 6-MHA played the role in this SAM. The cyclic
voltammetric curves in Fig.2 showed that the capacitance current of gold electrode
decreased after modified by 6-MHA. According to the equation
Cd = i/vS (1)
Here i is capacitance current; v is scan rate and S is the surface area
of electrode. The double layer capacitance of SAM modified electrode was then calculated[8]
for 18 m F/cm2, which is smaller than the bare gold electrode of 71
m F/cm2.

Fig.2 The CV curves of (a) bare gold electrode and (b) SAM modified
electrode in 1.0mol/L H2SO4 solution. Scan rate: 100mV/s; real
surface area: 0.042cm2
3.3 Study of the electron transfer
property of 6-MHA SAM
A conventional Fe(CN)63-/Fe(CN)64- couplet was
selected as a probe to study the electron transfer property. Its cyclic voltammetric
responses on a bare electrode and the SAM electrode were different obviously (see Fig.3).
On the CV curve of the SAM electrode, the reduction and oxidation peak currents were both
smaller than that on bare electrode and the difference of peak potentials was much larger
than 60mV. It revealed that the electrochemical process on the SAM modified electrode was
not diffusion controlled.

Fig.3 Cyclic voltammetric curves of a bare electrode(a) and the
6-MHA/Au(b) in 1.0mol/L KCl solution containing 1.0mmol/L Fe(CN)63-.
Scan rate: 100mV/s
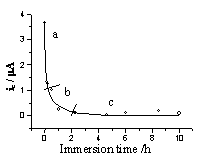
In the aforementioned supporting electrolyte solution, the peak current and peak potential of Fe(CN)63- on SAM electrode responded upon the self-assembling degree that was controlled by immersion time of electrode in 6-MHA solution. The relative curves were showed in Fig.4 and Fig.5.
 |
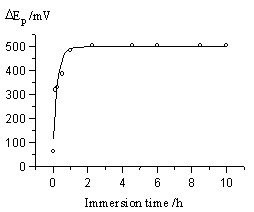 |
| Fig.4 The response of cathodic peak current of Fe(CN)63- upon the self-assembling degree of the electrode. | Fig.5 Relation between peak potential difference and the immersion time |
¡¡From the above two curves, the
self-assembling process could be divided into three periods. The first period (part a in
Fig. 4) is a fast adsorption process for about thirty min. In this process, the peak
currents decreased rapidly with the increase of dipping time due to the strongly
affinitive action between S and Au atoms. The formation of S-Au bond brought about the
adsorption of 6-MHA on gold electrode surface. This process would complete within about 30
minutes and obeyed the adsorption equation[9]:
ln(1-q ) = -Kadct
(2)
Here q
is the coverage rate of adsorptive molecules on electrode surface, c is the concentration
in solution, t is the immersion time.
The line in Fig.6 proves the aforementioned recount. The average
adsorption rate of the electrode was calculated from the slope as 3.67¡Á10-3M-1s-1.
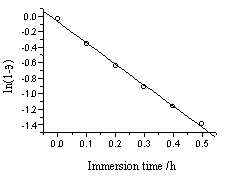
Fig.6 Relation between ln(1-q ) and the immersion time in first period of the adsorption process
The second period (part b
in Fig. 4) is a slower orientation and arrangement process of carbon chain. The decreasing
of peak current and the enlarging of peak potential difference got slow down gradually. It
needs 1.5 hours more. In this period, the molecules of 6-MHA had adopted an identical
orientation and formed a fine arrangement.
The third period is in equilibrium state. After immersing the gold
electrode into 6-MHA¡¯s acetone solution for two
hours, the self-assembled process would achieve the equilibrium state. The peak current
and peak potential difference did not change any more. The coverage of SAM was calculated
for 96% in this state by the results of coulometry of bare electrode and SAMs modified
electrode in Fe(CN)63-/Fe(CN)64- solution.
From the previous discussion about the SAM, the mechanism of redox
reaction of Fe(CN)63-/Fe(CN)64- could be
considered that the diffusion controlled process was replaced by an electron transfer
process after the formation of SAM. The 6-MHA SAM had obstructed the electron transfer
process between the gold electrode and probe couplet. It can be explained with a tunneling
process[10] that the electrons passed through the SAM via a tunnel, the
tunneling coefficient is 1.02¡À 0.2 per methylene. The apparent electron transfer rate
constants[11] have been calculated respectively for bare electrode of 9.13¡Á10-4
cm/s and 6-MHA SAM electrode of 2.47¡Á10-5 cm/s.
REFERENCES
[1] Sagiv J. J. Am. Chem. Soc., 1980, 102: 92.
[2] Nuzzo R G, Dubois L H, Allara D L. J. Am. Chem. Soc., 1990, 112: 558.
[3] Bryant M A, Pemberton J E. J. Am. Chem. Soc., 1991, 113: 8284.
[4] Hautman J, Klein M L. J. Chem. Phys., 1990, 93: 7483.
[5] Khim Y H, Field L. J. Org. Chem., 1972, 37: 2714.
[6] Gao P, Gosztola D, Leung L W et al. J. Electroanal. Chem., 1987, 233: 211.
[7] Gonez MM, Vazquez L, Salvarezza R C. J. Electroanal. Chem., 1991, 317: 125.
[8] Zhi Q F, Shinichiro I et al. J. Electroanal. Chem. ,1995, 394: 149.
[9] Kondo T, Takechi M, Sato Y et al. J. Electroanal. Chem., 1995, 381: 203.
[10] Dayton M A, Brown JC, Stutts K J et al. Anal. Chem., 1980, 52: 946.
[11] Xu J, Li H L, Zhang Y. J. Phys. Chem., 1993, 97: 11497.