http://www.chemistrymag.org/cji/2004/06b077ne.htm |
Nov. 1,
2004 Vol.6 No.11 P.77 Copyright |
Su Xiaohui, Gao Jungang, Jiang Chaojie
(Department of Polymer Science, College of Chemistry and Environmental Science, Hebei
University, Baoding 071002, China)
Keywords Phenol-formaldehyde resin, Boric acid, Nitrogen, thermal analysis
1. ITRODUCTION
Phenolic formaldehyde resins (PFRs) are used principally in the reinforced thermosetting
materials. To improve the flame retardancy and thermo-oxidative resistance of PFR, the
addition of boron has been reported [1-4].The boron- containing
phenol-formaldehyde resin (BPFR) is a modified phenolic resin, which is obtained by
introducing boron to the main chain of common phenol formaldehyde resin. The BPFR
possesses many excellent performances, such as thermostability, mechanical strength,
electric properties and defence of neutron radiation. But the general BPFRs are extremely
sensitive to moisture. To improve the hydrolytic resistance of BPFR, the resins of B-N
coordination bond have been synthesized [5,6], but the description of thermal
property and degradation kinetics has been lacking until now.
The glass transition temperature (Tg) can be used
effectively to illustrate the curing reaction process and thermal properties. In this
work, the BNPFR was synthesized, the torsional braid analysis (TBA) was used to determine
the Tg of the BNPFR, and thermogravimetric analysis (TGA) was used to
study the mechanism and kinetics of thermal degradation.
2. EXPERIMENTAL
2.1 Material
Phenol, boric acid, formaldehyde solution (37% w/w), oxalic acid, 25% ammonia
water, toluene, n-butyl alcohol and para-formaldehyde were all analytically pure
grades and were supplied by the Tianjin Chemical Reagent Co,China.
2.2 Synthesis of BNPFR
BNPFR used in this work was synthesized according to the literature [5,6],Changing
the ratio of boron and ammonia, the resins with different boron- nitrogen ratio were
obtained. Material proportion and the serial number were listed in Table 1.
Table 1 Material Proportion and Serial Number of BNPFR
| Composition | Phenol : Boric :
Ammonia (mol ratio) |
|
| Resin. | Common PR | 17 : 0 : 0 |
| 1.0N | 17 : 5.5 : 1.0 | |
| 2.2N | 17 : 5.5 : 2.2 | |
| 5.5N | 17 : 5.5 : 5.5 | |
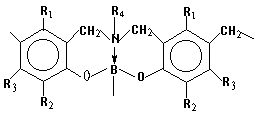
The molecular structure of
BNPFR has the following approximate form:
2.3 Torsional braid analysis (TBA)
Specimens, prepared by dipping heat-cleaned glass fiber braid in the tetrahydrofuran
solution of the BNPFR, were completely evaporated in vacuum.
1. The air oven was first heated up to a
desired temperature and kept for a certain period of time until the system reached the
equilibrium state. Specimens were quickly set into the thermostatic baths at a curing temperature
Tc between 140 and 220
2. Uncured resin was inserted into the GDP-4 TBA analyzer to determine the available curing temperature of different boron-nitrogen proportion resins.
2.4 TGA measurements
After curing at 180ºC for 4h, the thermal analysis was carried out on the Shimadzu DT-40 thermogravimetric analysis (TGA). About 8mg of the sample, which had been completely cured and ground to fine powers, was put into a platinum cell and placed on the detector plate, then the furnace was heated at a heating rate of 10ºC/min to 800ºC.
3. RESULTS AND DISCUSSION
3.1 Tg value and the curing process
Generally, the Tg of a
crosslinked resin system is related to the conversion, which depends upon the curing
conditions, such as temperature and time. With these curing condition varying, the Tg
of the system will be changed. So that Tg has been used
directly as a parameter for conversion analysis of curing reaction. Because of the Tg
of thermosetting resin is easy to be measured by TBA, it is particularly useful for the
measurement at high conversion and after vitrification. In this paper, the Tg
values are measured for the BNPFR specimen cured isothermally at different temperatures
for various periods. Different glass transition behaviors occurred for the 2.2N resin
samples with different degrees of curing temperature were shown in Table
2. As seen from Table 2, the BNPFR cured at lower temperature have a low Tg
compared with those cured at higher temperature. It is because that during the curing
process, cross linking reaction has occurred and the weight-average molecular weight
increases, the crossing linking density increases and the mobility of the chains
decreases, the chances for the molecules to collide with each other are reduced and the
intermolecular reaction at this low temperature is limited. But the samples have a higher
curing degree at higher temperature and the Tg value get higher. If the
resin was cured at 220
Table 2 The glass transition temperature of 2.2N resin curing at different temperature for 1h
| Curing temperature (ºC) | 140 | 160 | 180 | 200 | 220 |
| Tg (ºC) | 150 | 177 | 187 | 211 | £ |
To determine the influence of boron-nitrogen proportion on
Tgs, the TBA experiment of uncured resins were carried out, the results
are shown in Fig.1, where E is the modulus and F is the mechanical loss tangent. As seen
from Fig.1, at low temperature, the resin has not been cured, the modulus is low, and with
the temperature rising the resin begins to be cured, the modulus increases. When the
temperature approaches a finite value, the resin was completely cured, the modulus does
not change during a certain temperature range. From Fig.1 and Table 3, it can be seen that
there are the same Tg to BNPFRs of different boron-nitrogen proportion,
which illustrate that nitrogen content basically have no influence on Tg
of BNPFRs.
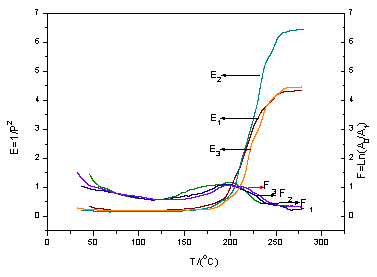
Fig.1 The TBA curve of BNPFR:
(1) 1.0N, (2) 2.2N, (3) 5.5N, at heating rate of 2ºC/min.
Table 3 the Tg of different resins cured at 200ºC for 1h
| Resin | 1.0N | 2.2N | 5.5N |
| Tg(ºC) | 214 | 211 | 214 |
It can be seen from Fig.2, that common formaldehyde resin (PFR) has higher weight loss rate than boron-nitrogen containing phenol-formaldehyde resin. The weight loss for common PFR is over 90% at 600ºC, while the BNPFR is only 32% at the same temperature. The start temperature of weight loss is at about 420ºC, higher than that of common PFR, and the temperature of half-weight loss is about 150ºC higher than that of PFR. It is due to the formation of phenol borate linkage, which reduces the formation of ether linkage, so that the heat- resistance of BNPFR has increased greatly.
As can be seen from Fig.2, the resins with B-N containing have the same weight loss before 320ºC, which is about 3%. We deem that it is caused by the evaporation of water and small molecules and it has not been considered as the degradation kinetics. The weight loss of BNPFR resins increased obviously from 420ºC, but the common resin decomposed distinctly from 320ºC, which indicates that the heat-resistance of common resin is inferior to BNPFR. According to the TGA curves (Fig.2), the degradation process can be divided into two stages. In the first stage (about 420-600ºC), the weight loss is about 20%, it may be caused by the oxidation and breakage of some ether linkages and carbonyl groups. In the second stage (about 600-800ºC), the weight loss is about 55%, it is due to most ether linkage, carbonyl group, some borate B-O linkage and benzene ring may be oxidized and broken in this stage.
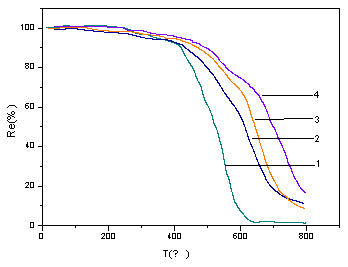
Fig.2 Thermogravimetric analysis of PFR (1) common PFR, (2) 5.5N, (3) 2.2N, (4) 1.0N, at heating rate of 10ºC/min in air.
To determine the kinetic
parameters of the decomposition from the thermo- gravimetric data, the first step is to
evaluate the conversion of the reaction. In dynamic TGA experiments, the weight change of
the sample is regarded as a function of temperature and the conversion can be expressed as
![]() Eq. (1)
Eq. (1)
where ![]() is the sample
weight in i stage,
is the sample
weight in i stage, ![]() is the residual
weight of
is the residual
weight of ![]() at temperature T.
Therefore, with TGA curves and Eq. (1), the conversions are calculated for different
degradation stages [7,8].
at temperature T.
Therefore, with TGA curves and Eq. (1), the conversions are calculated for different
degradation stages [7,8].
In this work, considering the multi-stage degradation process of BNPFR,
the TGA data were analyzed on the basis of the Madhusdanan-Krishnan-Ninan method [8],
which can be expressed by the following equation:
![]() Eq. (2)
Eq. (2)
Where A is the pre-exponential factor in the Arrhenius
equation, E is the apparent activation energy, R is the universal gas
constant, f
 . The correct
form of
. The correct
form of According to the above equations, the activation energy can be obtained at different heating rates from fitting
|
Reaction order | Correlation coefficient | Standard deviation | DE(kJ/mol) |
|
|
0.99435 | 0.14963 | 176.44 |
|
0.92303 | 0.34783 | 105.07 |
|
|
0.93041 | 0.6974 | 222.91 |
|
Second stage |
|
0.99339 | 0.18899 | 255.23 |
|
0.96389 | 0.23082 | 208.28 |
|
|
0.96613 | 0.46238 | 421.55 |
As shown in Table 4, for the same degradation stage at a given heating rate, the correlation values for different mechanisms are different. According to the principle that the probable mechanism has high correlation coefficient value and low standard deviation value, the probable mechanism functions are deduced from the calculated results: the two degradation stages are all following first order mechanism. The apparent activation energies in each stage are listed in Table 5.
Table 5 Apparent activation energy of thermal degradation
| DE(kJ/mol) | PFR | 5.5N | 2.2N | 1.0N |
| First stage | 130.04 | 153.48 | 176.44 | 279.93 |
| Second stage | 130.01 | 253.31 | 255.23 | 300.87 |
From the data above, we can conclude that heat-resistance of BNPFR is better than that of common PFR. The heat-resistance decreased step by step with the increase of nitrogen content, this is due to the increasing of C-N bond content, which has a lower heat-resistance than B-O bond.
4. CONCLUSION1. TBA can be used effectively to study the curing process of BNPFR and determine Tg.
2. The N content has little influence on Tg and the proper curing temperature of BNPFR is 200ºC. The Tg of BNPFR is at about 210ºC.
3. The modulus of the resin increases with the curing temperature increasing, and holding a certain value during a temperature range.
4. The thermal degradation process of BNPFR can be divided into two stages, and the thermal decomposition kinetics all follows the first reaction order.
5. The thermo-stability of BNPFR is better than common PR.
REFERENCES
[1] Gao J G, Liu Y F, Wang F L. Eur. Polym. J, 2001, 37: 207-210.
[2] Liu Y F, Gao J G. Polymer Degradation and Stability, 2002, 77: 495-501.
[3] Pitts A. Flame Retardancy of Polymeric Material. New York. Marcel Dekker. 1973.
chapter 2.
[4] Gerrard W. The organic chemistry of boron. London and New York: Academic Press, 1961,
chapter 17.
[5] Heefel H B, Kiessling HJ, Lamper F, Schonrogge B. Ger.Offen 2436358, 1975.
[6] Heefel H B, Kiessling HJ, Lamper F, Schonrogge B. Ger.Offen 2436359, 1975.
[7] Liu Z H. Introduction of thermal analysis. Beijing: Chemical Industry Publishing Co.
1991, 100-110.
[8] Madhusudanan P M, Krishnan K, Ninan K N. Thermochim. Acta., 1986, 97: 189-201.