http://www.chemistrymag.org/cji/2004/06b079ne.htm |
Nov. 1,
2004 Vol.6 No.11 P.79 Copyright |
Synthesis of new polymer-supported C2-symmetric
chiral
bis(oxazoline)s and their applications in asymmetric reactions
(Technical Center of Guangzhou No.2 Cigarette Factory, Guangzhou 510145, China)
Received on Aug. 23, 2004.
Abstract New solid Merrifield(3.8mmol
Cl/g) polymer-supported C2-symmetric chiral bis(oxazoline)s were
synthesized through a four-step sequence from diethyl malonate and chiral amino alcohols.
The catalytic activities of their Cu(I) complexes in cyclopropanation of
1,1-diphenylethylene with ethyl diazoacetate were studied. The highest enantioselectivity
of 85% ee and isolated 82% yield were achieved. The catalytic activities of the Cu(II)
complexes in ene-reaction of alpha-methylstyrene with ethyl glyoxylate solution were also
probed and enantioselectivity up to 50.8% ee and 89% yield were observed. Both in these
two catalytic reactions, the supported ligand 5a was the most effective one
compared with 5a and 5c. All the polymer-supported bis(oxazoline) ligands 5a-5c
could be easily recovered and were re-used in cyclopropanation and ene-reaction
respectively for additional two times with reaction yields comparable to that from a fresh
sample. However, enantioselectivity diminished considerably.
Keywords Polymer-supported, C2-symmetric bis(oxazoline), asymmetric
reaction£¬catalytic
C2-Symmetric chiral
bis(oxazoline)s have received much attention in recent years due to their notable
catalytic capabilities in enantioselective synthesis[1]. The Lewis basic
properties of the nitrogen donor atoms and the conformationally rigid frameworks in them
represent important structural features of this type of ligands. Although the
enantioselectivity of chiral bis(oxazoline)s are current subjects of intense research,
less is known about the efficacies of the ones in polymer-supported form[2].
It is well known, polymer-supported ligands have many attractive
advantages such as repeatable use, convenient separation and easy purification.
Accordingly, chiral bis(oxazoline)s 4a-4c were immobilized to solid polymers
and then the effectiveness of the resultant materials as catalysts for asymmetric
synthesis was examined. This article reports the synthesis of three new Merrifield
polymer-supported C2-symmetric chiral bis(oxazoline)s and the
application of their Cu(I) complexes in catalyzing the asymmetric cyclopropanation of
1,1-diphenylethylene with ethyl diazoacetate. The application of their Cu(II) complexes in
catalyzing the glyoxylate-ene reaction of alpha-methylstyrene with ethyl glyoxylate
solution were also probed. In order to assess the influence of the R group on the
stereoselectivity of the copper complexes-catalyzed cyclopropananation, three structurally
similar polymer-supported ligands 5a-5c were prepared. Different R groups
were easily installed by the use of different chiral b-amino alcohols as starting materials in a simple four-step
reaction sequence shown in Scheme 1.
The starting material for 5a, (-)-2-amino-1-butanol 1a,
is an inexpensive commercial available reagent; the other two amino alcohol 1b and 1c
were prepared from the corresponding a-amino acids, L-phenylalanine and L-valine by NaBH4-H2SO4
reduction[3]. The bis(oxazoline)s 4a-4c were obtained basically
according to the reported method[4]. Condensation of a-amino alcohol 1a-1c (2.2 eq.) with diethyl malonate
led to the dihydroxy malonodiamides 2a-2c. Appropriate amount of ethanol
could be added in the case of 1b in order to dissolve this solid material.
Treatment of 2 with methanesulfonyl chloride (3 eq.) and triethylamine (4 eq.) in
CH2Cl2 produced dichloro diamides 3a-3c which were
then treated with base to effect cyclization on the oxygen atom to form the
bis(oxazoline)s 4a-4c with quantitative yields. In contrast to what was
reported in the original literature method, the dichloro malonodiamides 3a-3c instead
of the corresponding bismesylates were isolated [4]. In the
experiments, it was noticed that compound 3a-3c underwent slow cyclization
on standing without added base to form 4a-4c as indicated by TLC. In the
presence of ethoxide base, 4a-4c were anchored onto Merrifield's resin
through their central methylene bridge to afford 5a-5c with retention of
their C2-symmetry. Results from both IR spectroscopy and elemental
analysis[5] showed that the degrees of functionalisation were 0.55, 0.82 and
0.60 mmol/g (resin) respectively.
Scheme 1

Scheme 2

Since the cyclopropanation of
1,1-diphenylethene with diethyl dizoacetate as the carbene source has been reported to be
effectively catalyzed by bis(oxazoline)-Cu complexes[6], this reaction
Table 1 Asymmetric cyclopropanation of 1,1-diphenylethene with ethyl diazooacetate using 4a-4c (6 mol%) and 5a-5c (6 mol%)
| Ligand | R |
Yielda(%) |
Enantioselectivity(%ee) |
Configurationb |
4a |
Et |
73 |
82 |
S |
4b |
CH2Ph |
47 |
76 |
R |
4c |
CH(CH3)2 |
51 |
81 |
S |
5a |
Et |
82 |
85 |
S |
5ac |
Et |
73 |
47 |
S |
5ad |
Et |
70 |
44 |
S |
5b |
CH2Ph |
52 |
70 |
R |
5bc |
CH2Ph |
54 |
42 |
R |
5bd |
CH2Ph |
53 |
40 |
R |
5c |
CH(CH3)2 |
51 |
78 |
S |
5cc |
CH(CH3)2 |
49 |
39 |
S |
5cd |
CH(CH3)2 |
50 |
37 |
S |
a) Isolated yield of 8 after column chromatography; b) Configuration determined by reported literature[6,7]; c), d) Upon re-using of catalyst for the first time and second time, respectively.
The data in Table 1
reveals that the reaction yield and the enantioselectivity are dependent upon the nature
of the bis(oxazoline) ligand. The polymer-supported ethyl-substituted ligand 5a and
4a perform well in the Cu(I)-catalyzed reaction, but better results in terms of
chemical yield and enantioselectivity were obtained with 5a. When the
polymer-supported ligands 5a-5c were recovered and re-used, the yield in
each case did not change noticeably except for 5a. However, enantioselectivities
were remarkably decreased compared with those from the corresponding fresh samples. The
reason why enantioselectivity decreased for the re-used catalyst samples is not clear yet.
General procedure for 8: 4 or 5 0.2 mmol, Cu(SO2CF3)2
0.2 mmol and CH2Cl2 5.0 mL were added into a flask in sequence
and refluxed for 0.5 h. Three drop of phenylhydrazine was then added and stirred for
several minutes. Excessive 1,1-diphenylethylene 11.0 mmol were added subsequently with
solution of ethyl diazoacetate 5.0 mmol and CH2Cl2 3.0 mL dropped
into slowly. 8 was obtained after 24 h, purified with column chromatography (100 :
1 petroleum ether and acetone as mobile phase).
Glyoxylate-ene reactions produce a-hydroxy esters, which are a class of compounds of synthetic and
biological importance. Annuniata et al[8] catalyzed the reaction (Scheme 3)
with the complexes of supported-MeOPEG Box and Cu(OTf)2 and achieved the
enantioselectivity up to 90 ee%. Qian et al£Û9£Ý
used Ph-pybox-Yb(OTf)3 to catalyze the
reaction and up to 49% ee were observed. Herein, the supported Cu(II) reagents 5a-5c
were applied for the first time to probe into their asymmetric catalyzing performance.
Scheme 3
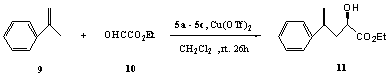
Table 2 Asymmetric glyoxylate-ene reaction of
alpha-methylstyrene with ethyl glyoxylate catalyzed by 5a-5c (6 mol%)
| Ligand | R |
Yielda(%) |
[ a ]tD (t)b |
Enantioselectivity(%ee) |
5a |
Et |
89 |
-19.3o(29.5ºC) | 50.8 |
5ac |
Et |
85 |
-11.2o(20.5ºC) | 29.5 |
5ad |
Et |
88 |
-5.5o(18.0ºC) | 14.5 |
5b |
CH2Ph |
87 |
-15.6o(18.8ºC) | 41.1 |
5bc |
CH2Ph |
83 |
-8.3o(18.2ºC) | 21.8 |
5bd |
CH2Ph |
79 |
-2.1o(13.0ºC) | 5.5 |
5c |
CH(CH3)2 |
88 |
-13.1o(22.5ºC) | 34.5 |
5cc |
CH(CH3)2 |
83 |
-7.3o(25.3ºC) | 19.2 |
5cd |
CH(CH3)2 |
84 |
-2.7o(23.8ºC) | 7.1 |
a) Isolated yield of 11 after column chromatography; b) CH2Cl2 as solvent; c), d) Upon re-using of catalyst for the first time and second time, respectively.
As shown in Table 2, the yield of 11 catalyzed by 5a was better than those of 5b and 5c, which is up to 89% ee. The yield of compound 11 catalyzed by re-used ligands was lower by 4-8% than those catalyzed by the fresh ligands. The enantioselectivity could be up to 50.8 ee% when catalyzed also by 5a. 5b came second, by which 41.1% ee could be attained. Compared with the initial use, the chiral catalyzing effect of 5a-5c decreased by 21.3%, 19.3% and 15.3% respectively when re-used for the first time, among which the ligand 5a remained the best one. When ligands 5a-5c were re-used for the second time, enatioselectivities decreased again to 14.5%, 5.5% and 7.1% compared with the second-time re-uses. In glyoxylate-ene reactions, the substituent R of the supported-ligand 5 effects the yield and enatioselectivity of the compound 11 to different extent. As to the enatioselectivity of the product, the result attained with R=ethyl is best. Benzyl comes second and iso-propyl comes third.General procedure for 11: 5 0.44 mmol and Cu(OTf)2 0.44 mmol were added into dry CH2Cl2 10 mL with the protection of N2. Refluxing for 0.5 h and cooled to 0ºC. a-methylstyrene and ethyl glyoxalate 22.5 mmol (50% toluene solution) were added in and reacted for 26 h. 11 was purified by column chromatography with acetone and petroleum ether 50 : 1 as mobile phase.
In summary, three new polymer-supported bis(oxazoline)s 5a-5c have been successfully synthesized, which proved to be efficient stereoselective catalysts for cyclopropanation and also glyoxylate-ene reactions. The highest enantioselectivity of 85% ee and isolated yield of 82% were achieved in cyclopropanation with polymer-supported bis(oxazoline) 5a. And enantioselectivity up to 50.8% ee and isolated yield up to 89% in glyoxylate-ene reaction were also achieved with 5a.
REFERENCES
[1] (a) For a general review of the use of chiral bis(oxazoline) metal in catalytic
asymmetric syntheses see: Ghosh A K, Mathivanan P, Cappiello J. Tetrahedron: Asymmetry
1998, 9: 1. (b) Lowenthal R E, Masamune S. Tetrahedron Lett., 1991, 32: 7373.
[2] (a) Hallman K, Moberg C. Tetrahedron: Asymmetry,
2001, 12: 1475. (b) Annunziata R, Benaglia M, Cozzi F, et al. J. Org. Chem., 2001, 66:
3160.
[3] Abiko A, Masamune S. Tetrahedron Lett., 1992, 33, 5517.
[4] Denmark S E, Nakajima N, Nicaise O J C, et al. J. Org. Chem., 1995, 60: 4884.
[5] All new compounds afforded satisfactory spectroscopic data as well as H1-NMR
and MS. Selected data for 5a: IR(neat): 3404, 3023, 2925, 1906, 1798, 1745, 1666, 1592,
1447, 1263, 1091cm-1; Anal. Calcd. for N: 6.41, Found: 1.53; 5b: IR(neat):
3436, 3056, 2918, 2853, 1942, 1904, 1812, 1739, 1603, 1493, 1448, 1352, 1263, 1098, 1018cm-1;
Anal. Calcd. for N: 4.99, Found: 2.30; 5c: IR(neat): 3435, 3023, 2924, 1906, 1605, 1510,
1447, 1369, 1263, 1094 cm-1; Anal. Calcd. for N: 5.59, Found: 1.67.
[6] Boulch R, Scheurer A, Mosset P, et al. Tetrahedron Lett., 2000, 41: 1023.
[7] Walborsky H M, Singh V K, Impastato F J. J. Am. Chem., 1961: 83, 2517.
[8] Annunziata R, Benaglia M, Cozzi F et al. J. Org. Chem., 2001: 66, 3160.
[9] Qian C T, Wang L. Tetrahedron: Asymmetry, 2000, 11: 2347.