http://www.chemistrymag.org/cji/2004/06b080pe.htm |
Nov. 1,
2004 Vol.6 No.11 P.80 Copyright |
Yin Pinghe,
Quan Changchun, Hong Aihua, Zhao Ling
(College of Life Science and Technology, Jinan University, Guangzhou 510632, China)
Keywords Waste edible oil collected from sewer; Volatile; GC-MS 1. INTRODUCTION
In many countries, restaurants would discharge the washing-water and remnant containing much oil into the sewer directly. The oil would float on the surface of sewer after the washing-water and remnant being discharged into sewer. It was reported that a little oil could increase COD and BOD rapidly, if the waste oil discharged into public sewer and public water bodies, it will result in the contamination of environment, so many countries have restricted the oil content of wastewater discharged into sewer[1,2]. For the environmental protection and economic profit, people would collect the waste oil floating on the surface of sewer and have it refined for other use. The way which the waste edible oil collect from sewer come from (WEOCFS) was showed in scheme 1:

WEOCFS could be used as materials of washing commodities, lubricant, cosmetic, biodiesel, and so on. The conversion of waste edible oil into biodiesel has drawn great attention of many researchers[3,4]. But some profiteers tempted by the high-profit would sell the refined WEOCFS in the normal edible oil market. The quality of oil in the sewer would be influenced by oxygen, sewage and microorganism greatly, for the oil would be oxidized by air contact, especially, this phenomenon is of particular interest in water-containing lipidic matrices[5]. Because the grease can be utilized as carbon source and energy by microorganisms, it is well known that fats and oils are readily degraded by a host of naturally occurring microorganism[2]. The consequence of oxidation and biodegradation is the development of unpleasant tastes and odors, characteristic of rancid fats and oils, as well as degradation of functional and nutritional properties and many of the degradation products of oils which are harmful to human health as they destroy vitamins, inhibit enzymes and potentially cause mutations or gastrointestinal irritaitions[6-8]. Moreover, some harmful components would be added to the WEOCFS in the process of refining for the removal of the water, odor and color. If being edible oil, the refined WEOCFS would be harmful to people's health.
Chinese media have reported the facts that many workshops had been producing refined WEOCFS and then selling them in the normal edible market illegally. People would get sick after consuming the refined WEOCFS. The events of WEOCFS drew great attention of society and government. The Ministry of Health, the State Commercial Administration of China, the State Environmental Protection Administration of China and the Ministry of Construction published the regulation together on the fifteenth of April 2002, forbidding the WEOCFS to be sold in normal edible market. In order to punish the profiteers and prevent WEOCFS from the edible oil market ultimately, we should make out the different components between WEOCFS and normal edible oils, and then distinguish the WEOCFS from normal edible oils. But there is few article concerned about it. In this article, we collected the volatile compounds by distillation, and determined the compounds by GC-MS, trying to find out the difference between WEOCFS and normal edible oils in some ways.
2. EXPERIMENTAL PROCEDURES
2.1 Materials
WEOCFS was obtained from the Environment Protection Administration of Yuexiu Guangzhou China. Hexane (A.R.) was purchased from Guangzhou Chemical Reagent Factory.
2.2 Collection of the volatiles
Because of the solvency of hexane for volatile compounds, it was used to collect the volatiles that had not been condensed in the first trap. The pressure of trap2 was 8.0¡Á104Pa.Oil sample (500g) was added into the flask(Fig. 1). This sample was purged with nitrogen for 15 min, and then was heated up to 100ºC at the rate of 5ºC/min, keeping the same temperature for 4h under a stream of nitrogen. The volatiles in trap1 and trap2, which were named as Volatile100, were mixed together and kept in refrigeratory for analysis by GC-MS. After the condenser and traps being changed, the sample was continued to be purged with nitrogen for 15 min and heated up to 100ºC quickly under the stream of nitrogen. The sample was heated up to 200ºC at the rate of 5ºC/min, keeping the same temperature for 4h.The volatiles in trap1 and trap2, which were named as Volatile200, were mixed together and kept in refrigeratory for the analysis by GC-MS. Another oil sample (500g) was added into the flask(Fig. 2), being purged with nitrogen for 15 min, and then the oil was heated up to 80ºC using water bath. After keeping the same temperature for 8h under the stream of nitrogen, 0.15g volatile (Volatile80) was got and kept in the refrigeratory for the analysis by GC-MS.
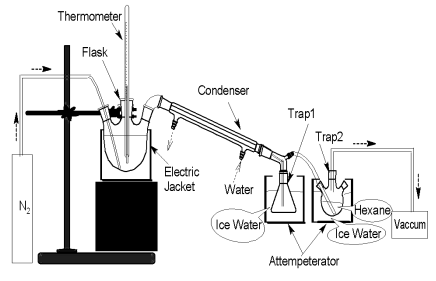
Fig.1 Apparatus for collecting Volatile100 and Volatile200
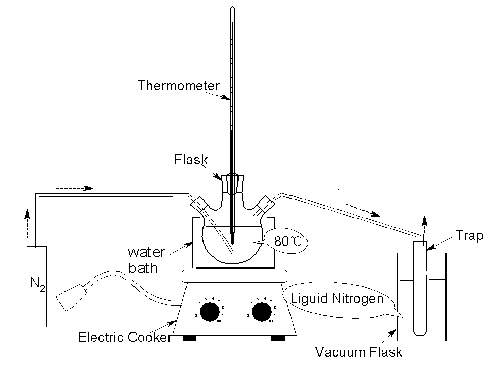
Fig.2 Apparatus for collecting volatile80 2.3 Analysis of the volatiles by GC-MS
The analysis of the volatiles of WEOCFS was performed by GC-MS (Trace, Finigan Co., U.S.A.). Separation of volatiles was achieved on a fused silica capillary column (B5; 30m¡Á0.24 mm ID¡Á0.25mm film thickness, J&W Science Folsom, CA).The helium carrier gas flow rate was 1.0mL/min, injector and detector temperatures were set at 200 and 250ºC, respectively. This mass spectrometer was operated in electron impact mode with an electron energy of 70eV and an emission current of 50¦ÌA. The ion source maintained at 200ºC. The mass spectrometer scanned from m/z 20 to m/z 300. The identification of volatiles was achieved by comparing mass spectrometer with those contained in the Nation Institute of Standards and Technology (NIST) Mass Spectral Database, and the semiquantitative analysis of these compounds was performed based on the peak area of these compounds.
Analysis of hexane: In order to observe the effect of purity of hexane to the volatiles, hexane was analysed by GC-MS. The oven temperature in the gas chromatograph was programmed as follows: the oven temperature was maintained at 60ºC for 2 min and then raised up to 120ºC at 5ºC/min rate with a final holding time of 3 min. The range of retention time is from 2 min to 16 min.
Anlysis of Volatile100: The oven temperature in the gas chromatograph was programmed as follows: the oven temperature was maintained at 60ºC for 2 min and then raised up to 120ºC at 5ºC/min rate with a final holding time of 3 min. The range of retention time is from 2 min to 16 min.
Analysis of the Volatile200: The oven temperature in the gas chromatograph was programmed as follows: the oven temperature was maintained at 100ºC for 2 min and then raised up to 200ºC at 5ºC/min rate with a final holding time of 3 min. The range of retention time is from 2 min to 34 min.
Analysis of the Volatile80: The oven temperature in the gas chromatograph was programmed as follows: the oven temperature was maintained at 30ºC for 2 min and then raised up to 100ºC at 4ºC/min rate with a final holding time of 3 min. The range of retention time is from 0 min to 10 min. 3. RESULTS AND DISCUSSION
3.1 Effect of purity of the hexane on the volatiles
When the retention time was more than two minute, there is no any peak in this chromatogram (seen from Fig.3) obviously, so the hexane was pure. It is unnecessary to consider the effect of impurity of hexane on the volatiles.
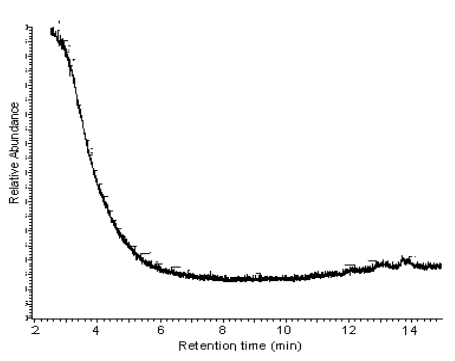
Fig.3 Total ion current chromatograph (TIC) of the impurity of hexane
3.2 Determination and semiquantitative
analysis of the Volatile80
Four compounds were detected in this sample(Table 1), two were aldehyde, the other was
alkanol, the last one was nitrogen that is the component of air. The highest content of
these volatiles was propanal, containing 69.84 percent.
Retention time |
Molecular weight |
M/Z |
Molecular formula |
Compounds |
Relative content (%) |
1.18 |
28 |
28 |
N2 |
Nitrogen |
4.64 |
1.28 |
44 |
29, 44 |
C2H4O |
Acetaldehyde |
21.85 |
1.46 |
58 |
29,45,58 |
C3H6O |
Propanal |
69.84 |
1.74 |
60 |
31,42,59 |
C3H8O |
Propan-1-ol |
3.66 |
3.3 Determination and semiquantitative
analysis of the Volatile100
Eight compounds were detected (Table 2). Six were alkanes, containing 85.88 percent;
another was alkanol, containing 0.50 percent; the last one was toluene, containing 13.62
percent. The highest content of these volatiles was octane, containing 66.63 percent.
Table 2 The volatile components of the waste edible oil collected from sewer heated at
100ºC analyzed by GC-MS.
Retention time |
Molecular weight |
M/Z |
Molecular formula |
Compounds |
Relative content (£¥) |
2.73 |
92 |
92,91,77,65,39 |
C7H8 |
Toluene |
13.62 |
3.08 |
114 |
114,85,71,57,43 |
C8H18 |
Octane |
66.63 |
3.59 |
130 |
112,83,82,71,57,41,28 |
C8H18O |
2-Ethylhexan-1-ol |
0.50 |
4.05 |
128 |
128,113,85,71,57,43 |
C9H20 |
2-Methyloctane |
1.47 |
4.65 |
128 |
128,99,85,71,57,43, |
C9H20 |
Nonane |
7.64 |
5.27 |
142 |
142,113,85,71,57,43 |
C10H22 |
3-Methylnonane |
0.90 |
6.51 |
142 |
142,99,85,71,57,43 |
C10H22 |
Decane |
6.58 |
8.43 |
156 |
156,113,98,85,71,57,43 |
C11H24 |
Undecane |
2.66 |
3.4 Determination and semiquantitative
analysis of the Volatile200
Twenty-seven compounds were detected (Table 3). Seventeen were alkanes, containing
17.90 percent; four were acids, containing 54.40 percent; two were benzene derivatives,
containing 2.07 percent; one was hexanal, containing 10.07 percent; one was alkene,
containing 0.32 percent; the last one was 2-pentyl-furan, containing 0.29 percent. The
highest content of these volatiles was oleic acid, containing 20.18 percent.
Table 3 The volatile components of the waste edible oil collected from sewer heated at 200ºC analyzed by GC-MS
Retention time |
Molecular weight |
M/Z |
Molecular formula |
Compounds |
Relative content(£¥) |
2.47 |
92 |
92,91,65,63,43 |
C7H8 |
Toluene |
0.88 |
2.78 |
100 |
85,82,72,57,56,44 |
C6H12O |
Hexnal |
10.07 |
3.70 |
128 |
128,113,106,91,71,57,43 |
C9H20 |
2-Methyloctane |
0.34 |
3.83 |
128 |
128,99,98,74,57,43,41 |
C9H20 |
3-Methyloctane |
0.32 |
4.32 |
128 |
128,99,85,71,57, 43 |
C9H20 |
Nonane |
1.09 |
5.00 |
102 |
73,60,57 |
C5H10O2 |
Pentanoic acid |
15.74 |
5.60 |
120 |
120,105,91,77,65,51 |
C9H12 |
1-Methyl-2-ethylbenzene |
1.19 |
5.75 |
142 |
142,112,105,71,57,43 |
C10H22 |
3-Methylnonane |
0.27 |
6.21 |
138 |
138,95,82,81,53, 28 |
C9H14O |
2-Pentylfuran |
0.29 |
6.39 |
142 |
142,113,99,85,71,57, 43 |
C10H22 |
Decane |
5.02 |
6.91 |
156 |
156,134,119,113,91,85,71,57,43 |
C11H24 |
4-Methyldecane |
0.54 |
7.08 |
136 |
136,121,107,94,93,79,68,67,53,39 |
C10H16 |
4-Isopropenyl-1-methylc-yclohexene |
0.32 |
7.71 |
156 |
134,105,99,91,85,71,57,43, |
C11H24 |
5-Methyldecane |
0.74 |
7.87 |
156 |
141,112,99,85,71,57, 43 |
C11H24 |
2-Methyldecane |
0.30 |
8.02 |
156 |
156,126,85,71,57,43 |
C11H24 |
3,7-Dimethylnonane |
0.17 |
8.72 |
156 |
156,113,98,85,71,57, 43 |
C11H24 |
Undecane |
3.14 |
10.02 |
170 |
152,112,85,73,71,57,43,41 |
C12H26 |
2,6-Dimethyldecane |
0.15 |
10.23 |
170 |
155,132,104,85,71,57,43 |
C12H26 |
2-Methylundecane |
0.17 |
11.08 |
170 |
170,146,131,99,85,71,57,43 |
C12H26 |
Dodecane |
2.46 |
11.40 |
184 |
145,99,85,71,57, 43 |
C13H28 |
2,6-Dimethylundecane |
0.20 |
12.75 |
184 |
160,113,97,85,71,57, 43 |
C13H28 |
2,6,7-Trimethyldecane |
0.04 |
13.37 |
184 |
184,112,99,85,71,57, 43 |
C13H28 |
Tridecane |
1.73 |
15.55 |
198 |
198,155,127,113, 99,85,71,57,43 |
C14H30 |
Tetradecane |
0.94 |
17.63 |
212 |
212,155,113,99,85,71,57,43 |
C15H32 |
Pentadecane |
0.43 |
26.29 |
256 |
256,227,213,199,171,157,129,115, |
C16H32O2 |
Palmitic acid |
17.88 |
29.01 |
282 |
264,180,137,123,98,97,93,69,55,41 |
C18H34O2 |
Oleic acid |
20.18 |
29.26 |
284 |
284,247,203,185,171,135,129,98, |
C18H36O2 |
Stearic acid |
0.45 |
In the study, thirty-two
compounds were detected, these included eighteen alkane-containing compounds, four
acid-containing compounds, three aldehyde-containing compounds, two benzene derivatives,
two alkanol-containing compounds, one alkene, and one 2-pentylfuran.
3.5 Discussion
Volatile compounds often come from the oxidative degradation of unsaturated fatty
acids, and are directly related to the structure of hydroperoxides, and their formation
depends on the nature of catalysis (enzymes, metals, light, heat). These compounds include
aldehyde, ketone, furan, alcohol, hydrocarbon etc, and the aldehyde was often the
predominant class of compounds[9,10]. But in the present study, hydrocarbon was
the predominant class of compounds, and only three aldehyde-containing compounds were
detected. Hexanal, which is the oxidation product of n-6 unsaturated fatty acids[11],
was detected in Volatile200; propanal, which is the oxidation production of n-3
unsaturated fatty acids[12]; was detected in Volatile80; acetaldehyde, which is
the second oxidation production of unsaturated fatty acids; was detected in Volatile80. No
any other familiar aldehyde, such as heptanal, nonanal, decanal, and some unsaturated
aldehyde, was detected in this study. The WEOCFS might be contaminated by mineral oil or
these aldehydes might have been biodegraded or undergone chemical decomposition reaction,
so the content of this kind of aldehydes was too low to be detected. But the reason why
the other familiar aldehydes can not be detected has not been understood completely and
would be studied further.
2-Pentylfuran was detected, which is the degradation production of
linoleate and showed a significant correlation with the time of oxidation[13].
The content of furan is often less than that of aldehydes in normal edible oil. No other
familiar aldehydes (except for hexanal) , while 2-pentylfuran could be detected in
Volatile200. So the WEOCFS might have undergone high oxidative reaction. This result
demonstrated the fact that the condition of sewer would accelerate the oxidative reaction
of oil and result in the degradation of oil's quality.
Acids are the products of oxidative decomposition of unsaturated fatty
acids and the hydrolysis products of triacylglycerides. Four acids were detected.
Pentanoic acid might be oxidized product. Palmitic acid, oleic acid and stearic acid are
the hydrolysis products of triacylglycerides[8]. In this study, content of free
fatty acids was very high(seen from Table3). This WEOCFS was further confirmed as the
highly hydrolyzed oil by the high acid value(AV), which is one unique characteristic of
WEOCFS, in another experiment of ours[14]. This result also demonstrated that
the condition of sewer would accelerate the hydrolysis of oil, which results in the high
content of free fatty acids.
Eighteen alkanes, from C8 to C15, were detected,
eight of which were straight hydrocarbons. It seems very different from other normal
edible oils. Some may be formed during the oxidation or thermal decomposition of fat[15,16,17,18].
Some may come from the biodegradation products of fat[2]. But most of
hydrocarbon may arise from the contamination, either from the sewer that some gas draff
may be discharged into by the cooks or from the refining process during which some mineral
oil may be added in. The reason why alkane was the predominant component is to be studied
further.
In conclusion, the volatile composition of WEOCFS seems great different from that of normal edible oils. The WEOCFS can also be known to have undergone high oxidative and hydrolyzed reaction by analyzing the component of volatiles. So we can distinguish WEOCFS from the normal edible oils by the volatiles, thus it affords a method for the effective administration of WEOCFS.
Acknowledgements This study was funded in part by National Science Fund of China(20277016) and National Science Fund of GuangDong(04105835).
REFERENCES
[1] Waken N G, Forster C F. Process
Safety and Environmental Protection: Transaction of the Institution of Chemical Engineers,
1998, 76 (1): 55-61.
[2] Kwaku Tano-Debranh, Seijiro Fukuyama, Naoki Otonari et al. Bioresource Technology,
1999, 69 (2): 133-139.
[3] Ayhan Demirbas. Energy Conversion and Management, 2002, 43 (17): 2349-2356.
[4] Y Zhang, M A Dubé, D D Mclean et al. Process design and technological
assessment, 2003, 89 (1): 1-16.
[5] N Frega, M Mozzon, G Lercker. Journal of the American Oil Chemists' Society, 1999, 76 (3): 325-329.
[6] Guillermo H, Crapiste, Marta I V, Brevedan, Amadia A. Carelli. Journal of the American
Oil Chemists' Society, 1999, 76 (12): 1437-1443.
[7] Yasushi Endo, Chang Mo
Li, Misako Tagiri-Endo et al. Journal of the American Oil Chemists' Society, 2001, 78 (10): 1021-1024.
[8] C P Tan, Y B Che Man. Food Chemistry, 1999, 67 (2): 177-184.
[9]Y.H.Hui, Bailey's Industrial Oil & Fat Products, Fifth Edition(1), Beijing: China
Light Industry Press, 2001.
[10] Anee Meynier, Claude Genot, Gilles Gandemer. Journal of the American Oil Chemists'
Society, 1998, 75 (1): 1-7.
[11]Kanner J, Harrel S, Hazan B. J. Agric. Food Chem. 1986, 34: 506-510.
[12] Frankel, E.N. Journal of the American Oil Chemist' Society, 1993, 70: 767-772.
[13] Stefania Vichi, Lorena Pizzale, Lanfranco S. Conte et al. J. Agric. Food Chem., 2003,
51 (22): 6564-6571.
[14] J Y Pan, P H Yin, H H Yu at al. Food Science, 2003, 24 (8): 27-29.(in Chinese).
[15] Mahinda Wettasinghe, Thava Vasanthan, Feral Temelli et al. Food Research
International, 2001, 34 (2-3): 149-158.
[16] D U Ahn, C Jo, D G Olson. Meat Science, 2000, 54 (3): 209-215.
[17] D V Byrne, W L P Bredie, D S Mottrdm et al. Meat Science, 2002, 61 (2): 127-139.
[18] D U Ahn, K.C. Nam, M. Du et al. Meat Science, 2001, 57 (4): 419-426.