http://www.chemistrymag.org/cji/2005/071009pe.htm |
Jan.21, 2005 Vol.7 No.1 P.9 Copyright |
Li Luhua, Zhou Jianke, Yue Qiang, Li
Jingxia
(Research Center of Physics and Chemistry Analysis, Hebei University; Hebei Province Key
Laboratory of Analytical Science and Technology, Baoding, 071002)
Received Nov.12, 2004.
Abstract A liquid chromatographic
method using indirect conductometric detection was proposed for the determination of
m-nitroaniline, ethanolamine, aniline, p-toluidine and triethylamine. Derivatization is
not required. The effect of composition of mobile phase including the organic
solvent、different conducting species and the
concentration of the chosen acid on detector response was studied. The column temperature
that affected the separation of ingredients was also studied. The calibration graphs
exhibited wide linear concentration ranges with correlation coefficients of better than
0.99 for all the components except for m-nitroaniline with correlation coefficients of
0.97. The detection limits were between 1.0mg/L-19mg/L. The method had been applied to
determine the amines in polluted water.
Keywords Ion chromatography; Indirect conductometric; amines
Amines have been widely used in oil
industry, food industry, agriculture and pharmacy. For example some components of crude
oil or natural gas, which can cause technical problems, require the use of amines or
amine-containing chemicals during production, transport or purification. But amines can
cause pollution and some serious diseases. Even more some amines may cause cancer. So
detection of amines has an important sense.
A number of methods had been proposed for the estimation of amines
including Gas Chromatography[1] and HPLC[2]. But the GC methods
often required derivatization before measurement and were technically tedious [3].
Ion-pair High-performance Liquid Chromatography could detected amines directly. But the
method existed some difficulties, for example such as interference of system peak[4].
Application for the determination of amines by IC have been known for many years[5-7].
A liquid chromatographic method using indirect conductometric detection was proposed in
recent years[8]. The basis of the detection was to create a conducting
background, which would decrease on elution of the organic compounds. Amino acids[8],
monosodium glutamate in foods[9] atropine and atropine alkaloids[10]
and active ingredients in cough-cold syrups[11] had been detected by indirect
conductometric. Our goal was to develop an alternative method for the determination of
aliphatic amines and aromatic amines. Some results of our works were summarized below.
1 EXPERIMENTAL
1.1 Apparatus
The LC system consisted of Shimadzu
Company HIC-6A Ion Chromatography with COD-6A conductivity detector, C-R5A integrator and SIL-6B auto injector. An analytical column from Alltech, Allsphere Cyano (CN)
(250mm×4.6mm i.d.) 5mm was used. The proposed mobile phase was water-methanol(65:35) containing 1.5mM toluene-p-sulfonic acid, other mobile
phases with different organic phases and acid were also studied as indicated in the
Results and Discussion.
Peaks were detected as negative changes in conductance, and the detector-integrator connections were reversed in polarity to give positive display of peaks on the integrator.
1.2 Reagents
m-Nitroaniline, ethanolamine, aniline, p-toluidine and triethylamine were all purchased from Acros Organics and were used without further purification. HPLC grade solvents were used to prepare the mobile phases. Deionized water obtained from a Milli-Q water purification system (Millipore).
1.3 Standard solutions and procedure
By dissolving the amines in and diluting them with water to the required concentrations except for m-Nitroaniline by diluting with methanol to the required concentrations standard solutions were prepared. The solutions were injected directly into the chromatograph. The signal integrator was activated immediately after each injection.
2 RESULTS AND DISCUSSION
2.1 Effect of the composition of mobile phase
The mobile phase was all-important for successful liquid chromatography. So organic phases were changed firstly under the conducting species fixed as perchloric acid, and then fixed the organic phases and conducting species as investigated in 2.1.1, 2.1.2 and 2.1.3.
2.1.1 The organic solvent
The retention times (tR) and capacity factors of the amines using different mobile phases were listed in Table 1.
Table 1 Effect of organic solvent on the retention times of the amines
Amines |
|
Capacity factor k' |
|||
A |
|
|
|
(mobile phase B) |
|
m-nitroaniline |
4.38 |
4.40 |
|
|
|
ethanolamine |
10.19 |
10.80 |
|
|
|
aniline |
11.70 |
12.54 |
|
|
|
p-toluidine |
13.55 |
14.76 |
|
|
|
triethylamine |
15.58 |
19.69 |
|
|
|
Mobile phase: A)
water-methanol-THF (67:30:3) with 1mM perchloric acid; B) water-methanol (65:35) with 1mM perchloric acid; C) water-acetonitrile (72:30) with 1mM perchloric acid; D) water-acetonitrile-THF (77:20:3) with 1mM perchloric acid.
It could be seen from Table 1 that mobile phases A, B and C could give
well resolved peaks within about 20 minutes for all the amines under study. Although
retention time of mobile phase B was longer than other mobile phases, methanol was more
cheap than acetonitrile and mobile phase B was more simple in composition than mobile
phase A.
2.1.2 The conducting species
The role of perchloric acid in the mobile phase is to create a conducting background to enable detection. So we studied on the different conducting species: trichloroacetic acid, toluene-p-sulfonic acid and acetic acid. It could be found that trichloroacetic acid and toluene-p-sulfonic acid could take place of perchloric acid and there was no response when using acetic acid. Toluene-p-sulfonic acid was chosen as the conducting species as it could give well resolved peaks within about 17 minutes for all the amines and was more safe and more stable than other acids.
2.1.3 The concentration of the conducting species
Our study on the retention behaviour of the amines indicated that the retention time decreased and the resolution value of the amines decreased with increasing in the concentration of toluene-p-sulfonic acid. Considered all the factors, toluene-p-sulfonic acid of 1.5mM was chosen as the conducting species. The mobile phase (water-methanol (65:35) with 1.5mM toluene-p-sulfonic acid) was chosen as the solvent system.
2.2 Effect of the column temperature
The temperature was changed from 32oC to 40oC. When the temperature was 38oC, it could be found that the retention time was shorter and column pressure was lower. So 38oC was chosen as the column temperature.
2.3 Separation of Standard solutions
The five amines were detected under the optimum conditions. The chromatograms of five amines were shown in Fig. 1.
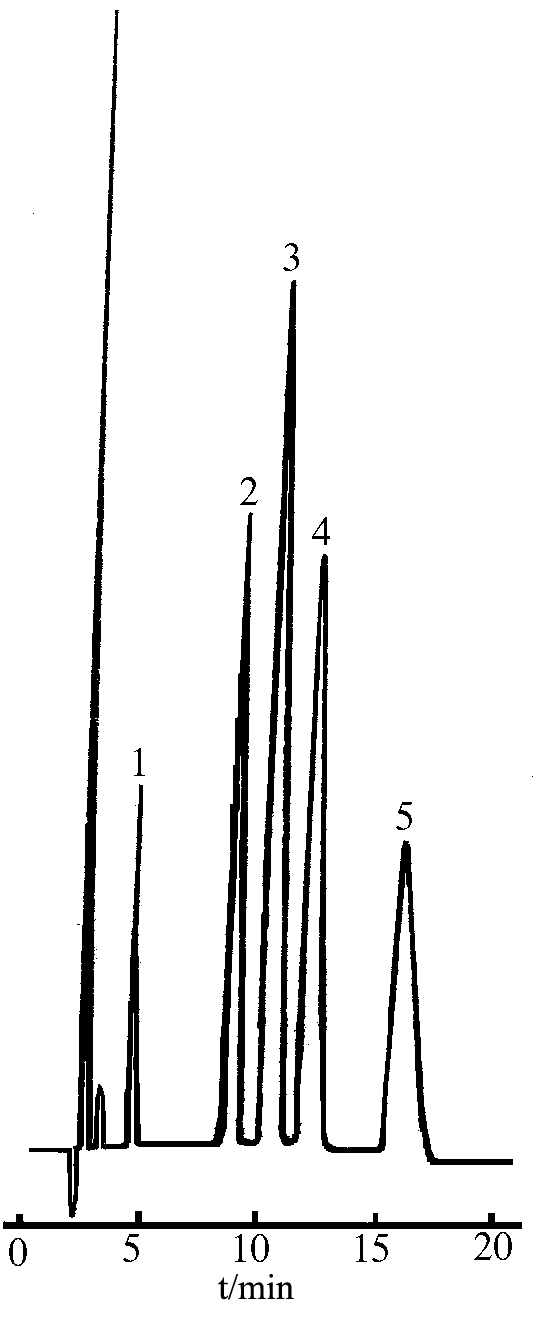 |
Fig.1 The standard
chromatogram of five amines |
2.4 Linear range and detection limit
The five amines samples with of different concentrations were detected under the optimum conditions. The calibration graphs of amines were found to be linear over the concentration range studied. The linear equations and the detection limits of the five amines were listed in Table 2. It could be seen from Table 2 that the peak area and the concentration of the detected sample exhibited well linear relation. And the calibration graphs exhibited wide linear concentration ranges with correlation coefficients of better than 0.99 for all the samples except for m-nitroaniline with correlation coefficients of 0.97. The detection limits were between 1.0mg/L-19mg/L.
Table 2 Linear equations and detection limits
Amines |
Linear equation |
Correlation coefficient |
Linear range (mg/L) |
Detection limit (mg/L) |
P (N=7) |
| m-nitroaniline | Y=2.14×104+8.21×104X |
0.9702 |
19-1.0×103 |
19 |
0.03 |
ethanolamine |
Y=3.78×103+9.4×105X |
0.9908 |
1.0-68 |
1.0 |
<0.0001 |
aniline |
Y=-1.04×102+4.28×105X |
0.9996 |
6.0-3.4×102 |
6.0 |
<0.0001 |
p-toluidine |
Y=-1.58×102+3.85×105X |
0.9999 |
6.0-3.1×102 |
6.0 |
<0.0001 |
triethylamine |
Y=-1.88×102+2.69×105X |
0.9998 |
7.0-3.6×102 |
7.0 |
<0.0001 |
Y-peak area; X-the concentration of the detected sample (mg/mL)
2.5 Interference study
2.5.1 Effect of filter
The peaks intensity of the five filtered standard amines were compared with that obtained by injecting the standard solutions directly into the chromatograph. It could be found that filter had little affection to detection.
2.5.2 Effect of inorganic ion and organic compound
The basis of the indirect conductometric detection was to create a conducting background, which would decrease on elution of the organic compounds. The conducting background would increase on elution of the inorganic ion, and it would give an opposite peak compared with analyzed substance. So inorganic ion had no affection to amines detection. The detector response was due to the conducting species absorbed on the stationary phase displacement by the amines. So the method had selectivity to amine and amine-like organic and other organic compounds like chloropropanols that we had experimented had no response.
2.6 Detection of the polluted water and recovery tests
The proposed method was applied to determine amines in polluted water sample. The polluted water (taken from protective river of Baoding) was filtered through 0.3mm filters and injected directly. It could be decided tentatively that the peak was ethanolamine's according to the retention time. The reliability of the method was studied by performing recovery test on a polluted water sample, where the peaks intensity of the polluted water sample that the five standard amines were added to were measured after running through the whole test procedure and compared with that obtained by injecting the polluted water sample directly into the chromatograph. Mean recoveries of three times of analysis for added amines were shown in Table 3. The concentration of ethanolamine was estimated according to the peak area of the standard sample and the polluted water sample. The concentration of ethanolamine in polluted water was 23 mg/L.
Table 3 Recoveries of the five amines
amines |
m-nitroaniline |
ethanolamine |
aniline |
p-toluidine |
triethylamine |
recoveries |
97.5% |
95.3% |
103% |
98.0% |
106% |
It is obvious that the method using a liquid chromatographic with indirect conductometric detection to determine amines provided an alternative method for the determination of amines.
REFERENCES
[1] Delport M, Maas S, van der Merwe SW. Journal of Chromatography B, 2004, 804:
345-351.
[2] Pielesz A, Baranowska I, Rybak A. Ecotoxicology and Environmental Safety 2002, 53:
42-47.
[3] Zhou Yuzhi, Shao Guangshao, Mu Shifen. Chinese Journal of Chromatography, 1977, 15
(3): 243-245.
[4] Viaue J, Navarro P, Naguyet T T. J Chromatogr, 1991, 549: 159.
[5] Rainer Kadnar. Journal of Chromatography A, 1999, 850: 289-295.
[6] Yao Tongwei, Zhang Guoxiang, Liu Zhiqiang. Journal of Zhejiang Medical University,
1992, 21 (1):
25-27.
[7] Zheng Demin. Natural Sciences Journal of Harbin Normal University, 2002, 18 (4):
67-70.
[8] Oi-Wah Lau, Chuen-Shing Mok Analytica Chimica Acta, 1995, 300: 183-191.
[9] Oi-Wah Lau, Chuen-Shing Mok Analytica Chimica Acta, 1995, 302: 45-52.
[10] Oi-Wah Lau, Chuen-Shing Mok Journal of Chromatography A, 1997, 766: 270-276.
[11] Oi-Wah Lau, Chuen-Shing Mok Journal of Chromatography A, 1995, 693: 45-54.
李路华 周建科 岳强 李敬霞
(河北大学理化分析中心,河北省分析科学技术重点实验室,保定 071002)
摘要 采用离子色谱间接电导法分离检测间硝基苯胺、乙醇胺、苯胺、对甲苯胺、三乙胺五种有机胺。探讨了不同流动相配比和不同的强酸高氯酸、三氯乙酸、对甲苯磺酸及其浓度变化对分离的影响,考察了不同柱温对分离的影响。方法不需衍生,被分析组份的峰面积与进样浓度呈良好的线性关系,除间硝基苯胺外线性相关系数可达0.99以上。各组分检测限范围在1.0mg/L-19mg/L之间。对污水进行了检测。
关键词 离子色谱法;间接电导;有机胺