http://www.chemistrymag.org/cji/2005/0710010pe.htm |
Jan.21, 2005 Vol.7 No.1 P.10 Copyright |
Degradation of rhodamine B in dilute aqueous solution with Ultrasonic Cavitation
Wang Xikui, Chen
Guanhong#, Guo
Weilin, Wang Jingang
(College of Chemistry and Environmental Science, Jinan University, Jinan 250022; #Biotechnology Research
Center of Shandong Academy of Science, Jinan, 250014)
Received Nov. 19, 2004; Support by the National Natural Science Foundation of China (20377019)
Abstract This paper presented the
research result of degradation of rhodamine B as a model of basic dye in aqueous solution
with Ultrasonic Cavitation. The ultrasonic degradation kinetics of rhodamine B in water
was found to be first-order and the degradation rate coefficient is 3.37×10-3
min-1 (R= 0.997, n=8) at 25±1℃. The
influence of the initial concentrations, reaction temperature, the pH of medium and Fenton
reagent on the ultrasonic decomposition of rhodamine B in water were also investigated.
Keywords Rhodamine B, degradation, ultrasonic cavitation, sonochemistry
Ultrasound is widely used for medical
imaging, cleaning and emulsification. Now ultrasound has different synthetic applications[1,2]
and has received as an advanced oxidation process for the decomposition of organic
pollutants in aqueous solution. The sonochemical reaction is regarded to originate from
acoustic cavitation. The cavitation processes consist of the creation, growth and
implosive collapse of gas vacuoles in a solution. According to the "hot spot"
theory [3], extreme temperature(-5000K) and high pressure(-1000atm) occur
within the bubbles during cavitational collapse. Under these extreme conditions, most of
organic compounds decomposed in the cavitation bubbles as well as compounds present in the
surrounding condensed layer undergo reactions comparable to those found in high
temperature combustion.
The ultrasonic degradation of a large number of chemical compounds of
environ- mental interest such as 1,1,1-trichloroethane [4], chlorinated
hydrocarbon [5,6], phenols [7,8] and surface active regents [9]
in aqueous solution have been studied. Dye is an important kind of environmental
pollutants in rivers or lakes. But there is a few reports on the sonolysis of dye in water
before [10,11]. In this paper we have studied the sonication of aqueous
solution of rhodamine B that was chosen as a model of basic dye.
1 EXPERIMENTAL
Aqueous solutions of rhodamine B (5.0 mgL-1 by mass) were prepared by
adding 5.0 mg rhodamine B to 1.0 L deionized water and stirring for 1 h. The sonication of
100 ml aqueous solutions of rhodamine B in a 150ml glass reactor cell were performed with
a 20 KHz Model JCS-204 Ultrasonic Reactor. The aqueous solution was saturated with pure
air before and during the sonication. The ultrasonic power was 50 w. The reaction
temperature was controlled with the help of condensation water surrounding the reactor
cell. The quantifications of rhodamine B were carried out with model UV-3000 photometer
(Shimadzu, Japan) and Model 244 HPLC (Backman, USA).
2 RESULTS AND DISCUSSION
2.1 Degradation kinetics
The investigation of the sonochemical degradation kenetics of rhodamine B were carried out
with 100 ml of air saturated aqueous solution (pH 5.20±0.2) at 25oC. During
the sonication, the concentrations of rhodamine B were determined every 30 min. The result
was showed in Figure 1. From the result it can be seen that the concentrations of
rhodamine B in aqueous solutions decreased exponentially with sonication time, indicating
first-order kinetics. Regression on the experiment data delivered the first- order
reaction rate coefficients is 3.37×10-3 min-1 ( r=0.997, n=8).
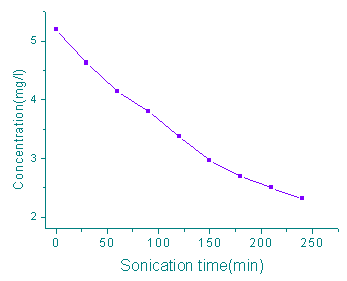
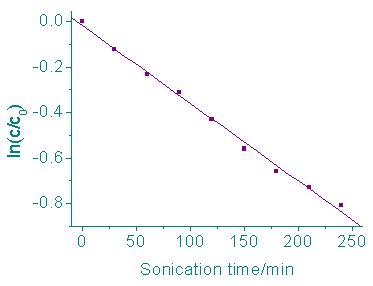
Fig. 1 Ultrasonic degradation kinetics of rhodamine B at 25oC
2.2 Effect of initial concentrations
The investigation of the effect of rhodamine B initial concentrations on the degradation
rate coefficients was carried out with 5-50 mgL-1 rhodamine B aqueous solution
at 25oC. It was found that the ultrasonic degradation rates of rhodamine B
varied at different initial concentrations. Table 1 showed the degradation rate
coefficients and the percentage of rhodamine B removed for 4 h at different initial
concentrations. It was observed that the degradation rate coefficients was decreased with
increasing of the initial concentration of rhodamine B. This could be explained by
surmising that the degradation intermediates of rhodamine B compete for the ·OH
radicals with rhodamine B itself, and some of intermediates could diffuse into the
cavitation bubbles changing the condition of bubbles collapse[7]. As a result,
the more intermediates that were formed and then oxidized by ·OH radicals, the
greater is the decrease in rate coefficients of rhodamine B degradation.
Table 1 The degradation rate coefficients (k1) and the percentage of rhodamine B removed for 4 h at different initial concentrations (25oC, pH=5.2)
Initial concentrations / (mg L-1) |
5 |
10 |
20 |
50 |
k1×103/( min-1) |
3.37 |
2.80 |
2.01 |
1.40 |
r |
0.997 |
0.998 |
0.998 |
0.999 |
% rhodamine B removed |
56.1 |
49.3 |
39.5 |
28.6 |
2.3 Effect of temperature
The influence of the temperature of the reaction solution on the ultrasonic
degradation of rhodamine B was investigated and the result was showed in Figure 3. It was
found that the ultrasonic degradation rate coefficients of rhodamine B were 3.37×10-3,
2.72×10-3 , 2.20×10-3 and 1.41×10-3 min-1
at temperature 25±1oC, 35±1oC, 45±1oC and 60±2oC respectively.
The result showed that with increasing the reaction temperature, the rate of degradation
was decreased in the range of temperature in this study. It has been reported that three
different regions are formed in the aqueous sonochemical process [12]: (1) The
gas phase within the cavitation bubble where elevated temperature and high pressure are
produced, (2) The interfacial zone between the bubble and the bulk solution where the
temperature is lower than that inside the bubble but still high enough for a sonochemical
reaction. (3) The bulk solution at ambient temperature where reaction still takes place.
Of the aforementioned three regions, we prefer the interfacial zone as the region where
rhodamine B was destructured because of the low vapor pressure of the compound. As the
bulk temperature of water increased, the vapor pressure of water and volatile solutes
inside the cavitation bubbles is increased. The collapse of cavity is thus cushioned more
than that at a lower bulk temperature, this results in more moderate conditions and a
lower sonochemical degradation rate.
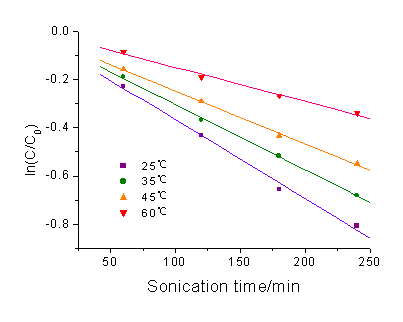
Fig. 3 Ultrosonic degradation kinetics of rhodamine B at different
temperature
2.4 Effect of the acidity of the medium
The acidity of the medium, which results in modification of the physical properties of
molecules with ionisable functional groups, plays an important role in sonochemical
degradation of chemical pollutants. The influence of the acidity of the reaction medium on
the ultrasonic degradation of rhodamine B was also studied and the results was showed in
Table 2. During sonication the buffers limited the pH-variation to less than 0.2 pH-unit.
So there is an influence of pH on the degradation rate of rhodamine B. It was found that
the ultrasonic degradation of rhodamine B was strongly dependent on pH. The degradation
rate coefficients and rhodamine B removed for 4 h in acidic water(pH 2-4) are higher than
those obtained in neutral aqueous solution (pH 5-10), and higher than also obtained
at basic medium.
Table 2 The degradation rate coefficients (k1)and the percentage of rhodamine B removed for 4 h at different pH (5 mg L-1 , 25oC)
pH |
2.05 |
4.00 |
5.20 |
8.05 |
10.00 |
12.10 |
k1×103/( min-1) |
10.03 |
5.33 |
3.37 |
3.71 |
3.90 |
5.56 |
r |
0.998 |
0.999 |
0.997 |
0.998 |
0.998 |
0.999 |
% rhodamine B removed |
90.4 |
72.1 |
56.1 |
59.5 |
62.8 |
73.9 |
2.5 Effect of free radicals
accelerator
It is well known that in water ultrasound can cause the formation of OH radicals which
are the very strong and nonspecific oxidizing species. Since OH radicals are major free
radical and important precursors for many products formed in sonolysis, the production
rate of HO· strongly influence the sonolysis efficiency. However, a lot of HO
radicals generated in cavitation bubbles would combine each other to form H2O2
in water and lead to the decreasing of the sonolysis efficiency. Many efforts have
therefore been devoted to improve the efficiency of sonochemical degradation[13-15],
particularly in view of the fact that a substantial amount of energy employed in
generating the radicals is not effectively converted into an optimum yield of the desired
products. A possible means of overcoming the generation of H2O2 is
to use it to produce additional ·OH radicals. According to Fenton reaction ,
Fe2+ can react with H2O2 to produce ·OH,
Fe2+ + H2O2 ---> FeOH2+ +
HO·
Fe3+ can be reduced by H2O2
via the formation of a Fe3+-hydroperoxy complex,
FeOH2+ + H2O2 ---> Fe(HO2)2+
Fe(HO2)2+ ---> Fe2+ + HO2·
So Fe2+ can
produce additional HO radicals and accelerate the sonolysis of pollutants. The effects of
Fe2+ on the ultrasonic degradation rate of rhodamine B was investigated with
sonication of 5 mgL-1 rhodamine B aqueous solution at pH 4.0 (25oC)
and the results were showed in Figure 3. It is found that the concentration of Fe2+
significantly affects the degradation rate coefficients of rhodamine B. The degradation
rate coefficients of rhodamine B increase with increasing initial concentration of Fe2+
in the range of concentration in this study.
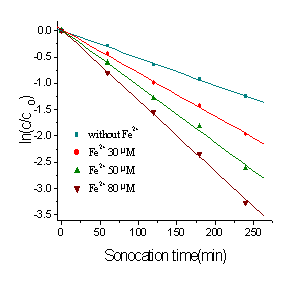
Fig. 4 Effect of Fe2+ concentration on
degradation rate of rhodamine B at 25℃(pH= 4)
3 CONCLUSION
It was found that the ultrasonic degradation kinetics of rhodamine B in aqueous
solution is first-order and the degradation rate coefficient is 3.37×10-3 min-1
at 25oC. It was observed that the degradation rate coefficients were decreased
with increasing of initial concentration of rhodamine B and reaction temperature. It was
found that the ultrasonic degradation of rhodamine B was strongly dependent on pH. The
degradation rate coefficients of rhodamine B in acidic or basic water are higher than
those obtained in neutral aqueous solution. The result also showed that Fe2+
can produce additional OH radicals and accelerate the sonolysis of rhodamine B in water.
REFERENCES
[1] Guo Wei Lin,Wang Xi Kui, Lin Zhi Ming et al.
Chem. J. Chinese Universities (Gaoxiao Huaxue Xuebao), 2002, 23 (8): 1592.
[2] Tatsuro M, Shinobu K, Hiroyasu N, Jpn. J. Appl. Phys., 2000, 39: 2902.
[3] Suslick K S, Doktycs S J, Flint E B, Ultrasonics, 1990, 28: 280.
[4] Gaddam, K, Cheung H M, Ultrasonics Sonochem., 2001, 8: 103.
[5] Petrier C, Francony A, Ultrasonics Sonochem., 1997, 4: 295.
[6] Drijvers D, Van Langenhove H, Herrygers V, Ultrasonics Sonochem., 2000, 7: 177.
[7] Jiang Y, Waite T D, Water Sci Technol., 2003, 47 (10): 85.
[8] Hua Inez, H?chemer R H, Hoffmann M R, Environ. Sci. Technol., 1995, 29: 2790.
[9] Yan Liu, Chinese J. of Applied Acoustics (Yingyong Shengxue), 1999, 18 (2): 35.
[10] Wang Xi Kui, Chen Guan Hong, Guo Wei Lin,Molecules, 2003, 8: 40.
[11] Wang Xikui, Guo Weilin, Yao Zhongyan et al. Environmental Chemistry (Huanjing
Huaxue), 2004, 23 (1): 105.
[12] Riesz P, Kondo T, Krishna C, Ultrasonics, 1990, 28: 295.
[13] Kang J W, Hung H M, Lin A et al. Environ. Sci. Technol., 1999, 33: 3199.
[14] Joseph J M, Destaillates H, Hung H M et al. J. Phys. Chem., 200, 104: 301.
[15] Weavers L K, Malmstadt N, Hoffmann M R, Environ. Sci. Technol., 2000, 34: 1280.
王西奎 陈贯虹# 国伟林 王金刚
(济南大学化学化工学院, 济南,250022; #山东省科学院生物技术研究中心)
摘要 本文研究了利用超声空化效应降解水溶液中微量罗丹明B的降解动力学和各种影响因素。结果表明,水溶液中微量罗丹明B可通过超声化学方法降解,降解动力学为一级反应,降解速率常数为-2.25×10-3 min-1 。罗丹明B的降解速率随初始浓度的升高而降低,随介质温度的下降而升高。罗丹明B的降解速率受介质酸度的影响较大,在中性介质中降解速率较低,而在酸性和碱性条件下降解速率显著提高。此外Fe2+能促进罗丹明B的降解。
关键词 罗丹明B,降解,超声空化效应,超声化学。