http://www.chemistrymag.org/cji/2005/073025ne.htm |
Mar.22, 2005 Vol.7 No.3 P.25 Copyright |
Zhou Guangyun, Yin Jingmei, Gao Dabin, Jia
Yingping, Wang Rui
(Liaoning Key Laboratory of Bio-Organic Chemistry, Dalian University, Dalian 116622)
Abstracts Nano cobalt is synthesized in
aqueous solution. Photopromoted carbonylation is made by olefins with CO and CO2
over nano cobalt catalyst under ambient conditions. It shows that the reaction can be
carried out very well and after the reaction the catalyst is easy to be separated and thus
can be reused for many times.
Keywords Photopromoted, carbonylation, nano cobalt, olefins, carbon monoxide,
carbon dioxide.
Carbon monoxide, carbon dioxide, (both of them 99.9%, Dalian Guangming Institute ); CH3OH, CH3COCH3 ( A.R., Dalian Merro Chemical Reagent and Glass Apparatus Co. ); n-decane ( A.R., Beijing Phentex Chemicals Co., Ltd. ); CoCl2·6H2O ( A.R., Shenyang Chemical Reagent Co. ); 1-octene ( 97%, Hong Kong Farco Chemical Supplies ), cyclohexene ( Military Medicine Academy of Sciences Material Supplies ).
2 PROCEDURE
A 1.6 mol/L NaBH4 aqueous solution was made from 1.5038 g NaBH4 and distilled water , which was purged with N2. A 1.0 mol/L CoCl2 aqueous solution was made from 2.0686 g CoCl2·6H2O, 1.5 ml polyglycol 600 and distilled water, which was purged with N2 for 20 minutes. The 1.6 mol/L NaBH4 aqueous solution was dropwise added to the 1.0 mol/L CoCl2 aqueous solution with stirring under N2 , and continued to react for 1 hour after the addition.
Then filtration was done and the filter cake was successively washed with distilled water, 1M HCl, distilled water and acetone (All of these solution were purged by N2). After drying under vacuum, 0.506 g nano cabolt was obtained with yield of 98.77%.
A typical carbonylation procedure was as follows: A quartz test tube with inner magnetic stirring (scheme 1) containing a solution of olefin ( 100 mmol/L ), catalyst ( 10 mmol/L ) and NaOAc(300mmol/L) in CH3OH-CH3COCH3 ( V:V=3:1, 15mL ) was irradiated for 20 hours using a high-pressure mercury lamp of 400W ( Philips & Yaming Corp. ) at room temperature under carbon monoxide or carbon dioxide at atmospheric pressure. The quartz test tube was placed closely against the outer wall of the quartz reactor with a length of 3.3×10-2m between the center of light and reaction solution, and illuminance of 2.6×10-2W/m2 . The products were detected by GC retention time and GC-MS.
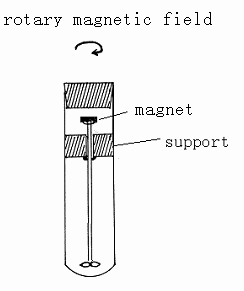 |
 |
| Scheme 1
The quartz test with magnetic stirring for carbonylation of olefins |
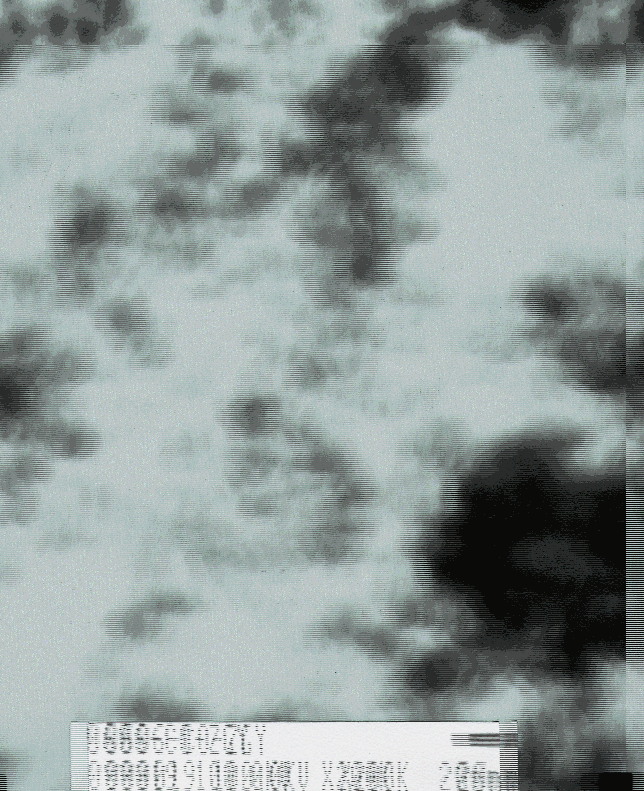
Scheme 2 TEM picture of nano cobalt |
Morphology of the obtained particles, which shows black and self-ignites in air, was observed with a JEM-2000EX 400T transmission electron microscopy (TEM) at 120kV and it is shown that the diameter of the particles is less than 20nm (Scheme 2).
The reactions of cyclohexene and 1-octene with CO and CO2 are as follows, and the results are shown in Table 1.
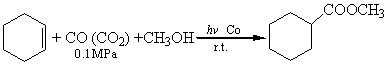
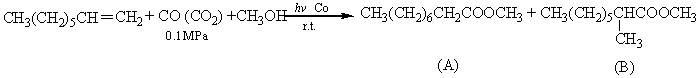
Substrates |
Carbon sources |
Yields (%) |
cyclohexene |
CO |
6 |
1-octene a |
CO |
27 |
cyclohexene |
CO2 |
3 |
1-octene a |
CO2 |
6 |
It can be seen that under
the catalysis of nano cobalt, photopromoted carbonylation of olefins can take place not
only with CO but also with CO2 at ambient conditions. The reactivity of CO2
is less than CO.
According to our previous work, NaOAc is beneficial to the reaction and
can improve the reaction activity[4]. The result of NaOAc as additives is shown
in Table 2.
Substrates |
Gas |
Yields (%) |
cyclohexene |
CO |
51 |
1-octeneb |
CO |
78 |
cyclohexene |
CO2 |
32 |
1-octeneb |
CO2 |
38 |
In Table 2 it is shown
that the yields of carbonylation of olefins is much higher after adding NaOAc. It has been
postulated that NaOAc as additives can modulate the pH of the reaction and thus can
improve the reactivity of carbonylation of olefins[4].
The research is also done of the carbonylation of olefins with CO and
CO2 in CH3OH under the catalysis of nano cobalt. The result is shown
in Table 3.
It can be seen from Table 3 that the yields of carbonylation of olefins
is lower in CH3OH than that in CH3OH-CH3COCH3,
and the carbonylation of olefins with CO2 can not take place in CH3OH,
which means that CH3COCH3 is necessary to the carbonylation of
olefins with CO2 but not necessary for carbonylation of olefins with CO, which
is not coincident with the references[5].
Substrates |
Carbon sources |
Yields (%) |
cyclohexene |
CO |
12 |
1-octenea |
CO |
51 |
The preliminary research is made of the recycle of nano cobalt as catalysts through the carbonylation of cyclohexene with CO2 in CH3OH-CH3COCH3. The comparison is made with solvent containing a small amount of water and with dried solvent. The result is shown in table 4.
Table 4 The Yields of Recycle of Nano Cobalt as Catalysts for Carbonylation of cyclohexene in CH3OH/CH3COCH3Run |
1st |
2nd |
3rd |
4th |
5th |
1a |
23 |
23 |
8 |
0 |
0 |
2b |
32 |
32 |
26 |
23 |
19 |
a: Solvent (not removing water). b: Solvent (removing water)
It can be seen that the
existence of a small amount of water is harmful to the recycle of nano cobalt in
photopromoted carbonylation of cyclohexene.
IR and TCD are used to study carbonylation of olefins with CO2
in CH3OH-CH3COCH3. CO is found by IR in CH3OH-CH3COCH3,
but not in CH3OH. 13CO2 isotope experiment shows that CO2
takes part in the reaction, which is supposed that CH3COCH3 is
beneficial to activate CO2[6].
Acknowledgment
We are indebted to the National Natural Science Foundations of China (No. 20242004,
20372012) and Foundations of Key Laboratory of Photochemistry, Center for Molecular
Science, Institute of Chemistry, Chinese Academy of Sciences for the generous financial
support.
[1] Othman H, Arab E, Patrick M H. J. Org. Chem., 2001, 66: 180.
[2] Yin J M, Gao D B, Wang R et al.. Chinese Chem. Lett., 2000, 11 (3): 223.
[3] Gao D B, Yin J M, Guo M et al.. Chinese Chem. Lett., 1998, 9: 423.
[4] Gao D B, Yin J M, Guo M et al.. Chinese Journal of Catalysis, 1999, 20 (3): 290.
[5] Tao Y T, Yeh W L, Tu C L, Chow Y L. J. Mol. Catal., 1991, 67 (1):105.
[6] Yin J M, Gao D B, Hu J H et al.. Chinese Science Bulletion, 2003, 48 (20): 2180-2182. 光促进下纳米钴催化的烯烃羰基化反应
周广运 尹静梅 高大彬 贾颖萍 王锐1
(大连大学生物有机省重点实验室,辽宁大连, 116622)
摘要 用液相法合成了纳米钴。研究了纳米钴在光促进常温常压下催化烯烃与一氧化碳或二氧化碳的羰基化反应。实验结果表明,纳米钴的催化效果较好,反应后催化剂纳米钴易于分离,可重复使用。
关键词 光促进 羰基化反应 纳米钴 烯烃 一氧化碳 二氧化碳