http://www.chemistrymag.org/cji/2005/073026ne.htm |
Mar.22, 2005 Vol.7 No.3 P.26 Copyright |
You Mingge, Tang Yu, Zhang Yuanming
(Dept. of Chemistry , Jinan University , GuangDong Guangzhou 510632,China)
Abstract The effects of phosphotungstic
acid amount and reaction time on preparation of 2-tert-butylhydroquinone (TBHQ) from
2,5-di-tert-butylhydroquinone (DTBHQ) were discussed.The good yield of TBHQ was 74.0% with
DTBHQ conversion of 43.1%, when 20g of DTHBQ was refluxed in 100ml of xylene for 2 hours
catalyzed by 1.5g of phosphotungstic acid. The effects of crystal water on catalyzed
reaction were investigated under the conditions of dried phosphotungstic acid, dried
phosphotungstic acid mixed with water before and after the other reagents were mixed
together. The result showed that the reaction is affected by crystal water through
affecting the acidity of phosphotungstic acid. The mechanism of DTBHQ dealkylation was
also discussed.
Keywords 2-tert-butylhydroquinone; 2,5-di-tert-butylhydroquinone; phosphotungstic
acid
1 INTRODUCTION
2-tert-butylhydroquinone (TBHQ) is an antioxidant widely used in foods, cosmetics and
rubbers, especially in oils and fats. [1,2] It can be made from hydroquinone by
alkylation with tert-butanol. The by-product 2,5-di-tert-butylhydroquinone (DTBHQ) is also
produced in the preparation of TBHQ. So the yield of TBHQ was low to about 30%.[3]
Although the DTBHQ is also an antioxidant, it has not been used widely, and can not be
sold very well. We have developed a method to synthesize TBHQ from DTBHQ catalyzed by
p-toluenesulfonic acid.[4] We tried to proceed the reaction by using sulfuric
acid, phosphoric acid, oxalic acid, citric acid, acetic acid and phosphotungstic acid. But
only sulfuric acid and phosphotungstic acid could catalyze the reaction. The catalytic
effect of sulfuric acid was not good for its too strong acidity. The better yield of TBHQ
was 74.0% with DTBHQ conversion of 43.1%, when 20g of DTBHQ was refluxed in 100ml of
xylene for 2 hours catalyzed by 1.5g of phosphotungstic acid. This result was a little
better than our former work..[4] The effect of crystal water on reaction
through affecting the acidity of phosphotungstic acid was investigated. The unique role of
crystal water of phosphotungstic acid in gaining good yield of TBHQ was also pointed out.
A reasonable mechanism of DTBHQ dealkylation was deduced from the experimental results.
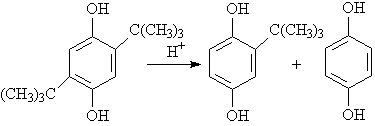
Scheme 1
2 EXPERIMENTAL
2.1 Materials and instruments
DTBHQ was an industrial product and purified by the method of literature 4. The other
reagents were analytical or chemical grade.
WRS-1 digital melting point apparatus, EQUINOX 55 spectrometer for
IR(KBr), Finnigan TRACEGC-MS spectrum.
2.2 Preparation of TBHQ from DTBHQ
20g of DTBHQ, 100ml of xylene, some phosphotungstic acid and water were placed
together. The mixture was refluxed for some hours. After cooled to room temperature, the
mixture was placed in refrigeratory for crystallization. The crystal was filtrated and
washed with cool xylene. Then the dried solid was dissolved in 80ml hot water. The hot
solution was filtrated, and the insoluble DTBHQ solid was collected. After the filtrated
water solution cooled, the TBHQ crystal was filtrated and washed with cool water.
3 RESULTS AND DISCUSSION
3.1 The effect of phosphotungstic acid amount
From the results in Table 1, the better yield of TBHQ was 74.0% when the conversion of
DTBHQ was 43.1% catalyzed by 1.5g of phosphotungstic acid. Compared with the better result
catalyzed by p-toluenesulfonic acid, the conversion of DTBHQ decreased 5.0% from 48.1%,
but the yield of TBHQ increased 6.4% from 67.6%. [4] Furthermore, the usage of
phosphotungstic acid was less than 2.0g of p-toluenesulfonic acid. On the other hand,
phosphotungstic acid could be immobilized more easily than p-toluenesulfonic acid. [5]
This endues it a good application future by used repeatedly. So the result in this paper
could be said better than our former work by general consideration. The reaction still
showed that too much acid promoted the conversion of DTBHQ and yielded less TBHQ, while
too less acid made both conversion of DTBHQ and yield of TBHQ low.
Table 1 The effect of the phosphotungstic acid amount on the yield of TBHQ.*
Phospho tungstic acid(g) |
0.5 |
1.0 |
1.5 |
2.0 |
2.5 |
3.0 |
YDTBHQof conv. (%) |
38.0 |
39.8 |
43.1 |
46.2 |
55.9 |
65.0 |
YTBHQ (%) |
53.3 |
62.5 |
74.0 |
61.4 |
32.2 |
5.9 |
*Yield of TBHQ was calculated on the amount of consumed DTBHQ in reaction. Yield of DTBHQ conversion was calculated on the amount of DTBHQ charged in reaction.
3.2 The effect of reaction time
The effect of reaction time was discussed under the condition of 1.5g of
phosphotungstic acid. The results were shown in Table 2. The same as the result in our
former work, the better reaction time was still 2 hours. High conversion of DTBHQ but low
yield of TBHQ was gained by long time reaction.
Table 2 The effect of reaction time on the yield of TBHQ.
Reaction time(h) |
1 |
2 |
3 |
4 |
5 |
6 |
YDTBHQof conv. (%) |
32.1 |
43.1 |
59.3 |
73.3 |
73.9 |
92.0 |
YTBHQ (%) |
54.1 |
74.0 |
43.6 |
41.4 |
39.1 |
23.2 |
3.3 The effect of water
The yield of TBHQ could be improved by addition of water in our former work [4].
So we tried to add water in the reaction under the condition of refluxing 2 hours
catalyzed by 1.5g of phospho- tungstic acid. But both the yield of TBHQ and conversion of
DTBHQ decreased by even a very few addition of water. Apparently, the catalysis of
phosphotungstic acid became weak. The same possible reason as p-toluenesulfonic acid [4]
was that phosphotungstic acid had already contained crystal water, and would become less
dissolution in reaction by adding more water. The results were shown in Table 3.
Table 3 The effect of water amount on the yield of TBHQ
Water(ml) |
0.05 |
0.10 |
0.20 |
0.30 |
0.40 |
YDTBHQof conv. (%) |
36.50 |
47.35 |
32.90 |
23.45 |
13.65 |
YTBHQ (%) |
47.08 |
38.90 |
27.43 |
14.31 |
6.81 |
In order to discuss the effect of crystal water on the reaction, phosphotungstic acid was desiccated to constant weight at 120℃(losing 11.4% weight of water). Then the dried phosphotungstic acid was applied to catalyze the reaction by refluxing for 2 hours. The results were shown in Table 4. The conversion of DTBHQ was improved from 38.0% to 93.15% by 0.5g of dried phosphotungstic acid. But the 92.0% and 65.0% conversions of DTBHQ could only be gained by 1.5g of phosphotungstic acid with crystal water after reacting 6 hours and by 3.0 g of phosphotungstic acid with crystal water after reacting 2 hours respectively. Obviously, the catalytic potence of phosphotungstic acid had been improved by losing water. The other evidence was the only 1.0% yield of TBHQ, that was to say the TBHQ had also been changed to hydroquinone by a strong catalyst. So the water was very important for the reaction.
Table 4 The effect of dried phosphotungstic acid on the yield of TBHQ and conversion of DTBHQ.
Dried phospho tungstic acid(g) |
0.50 |
1.50 |
2.50 |
YDTBHQof conv. (%) |
93.15 |
99.10 |
96.75 |
YTBHQ (%) |
1.00 |
0.29 |
1.72 |
In order to discuss the effect of water more deeply, the reaction was proceeded by mixing dried phosphotungstic acid with water before and after the other reagents were placed in reaction. The results were shown in Table 5. The conversion of DTBHQ decreased by adding water to the reaction, while the yield of TBHQ increased to a relative high value, but decreased by adding more water. The rule was the same as the reaction catalyzed by original phosphotungstic acid with crystal water.
Table 5 The effect of water amount on catalytic reaction of dried phospho tungstic acid
Water amount(ml) |
0.05 |
0.08 |
0.10 |
0.12 |
0.15 |
0.20 |
YDTBHQof conv.(%)1) |
99.3 |
79.5 |
44.1 |
23.6 |
20.7 |
11.0 |
| YTBHQ(%)1) | 1.6 | 42.1 | 49.2 | 23.7 | 17.8 | 17.5 |
YDTBHQof conv.(%)2) |
99.5 |
67.80 |
46.7 |
26.6 |
24.9 |
21.0 |
| YTBHQ(%)2) | 3.7 | 47.66 | 55.9 | 48.5 | 39.7 | 30.8 |
1)Water was mixed with dried phosphotungstic
acid after other reagents.
2) Water was mixed with dried phospho tungstic acid before the other reagents.
Compared this three reaction ways, the general effect sequence was original phosphotungstic acid with crystal water >the way of premixing water>the way of mixing water later. This could also be the effect sequence of combining water with phosphotungstic acid to form crystal water. The acidity of phosphotungstic acid could be improved by evaporating its crystal water through heating.[6,7]It could explain our experimental results preferably. The dried phosphotunstic acid with a stronger acidity was also a powerful catalyst. It could change both DTBHQ and TBHQ to hydroquinone, so the conversion of DTBHQ was high, but the yield of TBHQ was low. Although water was mixed with phosphotungstic acid in different ways, it could decrease the catalytic ability by reducing the acidity of phosphotungstic acid. The same results were obtained with other acids, the conversion of DTBHQ was 98.9%, but the yield of TBHQ was only 2.1% when catalyzed by 0.3ml of strong sulfuric acid, and there is no reaction when catalyzed by weak acids such as oxalic acid, citric acid and acetic acid.
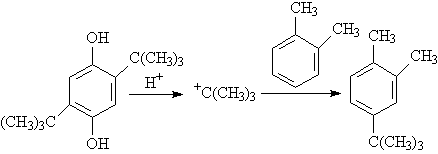
Scheme 2
3.4 The mechanism of dealkylation of DTBHQThe t-butyl xylene such as 4-t-butyl-o-xylene[MS(m/s):163(9),162(35),147(100),119(58), 107(17,77(8),65(5),51(3)) was detected in the filtrated reaction solution by GC-MS. So a possible mechanism through carbon cation was put forward as shown in Scheme 2. In a similar reaction, the Carbon cation was detected in the dealkylation of 2,5-di-t-butyl-p-dimethoxybenzene[8].Through this mechanism, it is easier to attack the benzene ring for a strong acid to form t-butyl cation. It could be shown by reactions promoted by strong acids. The dealkylation of DTBHQ is the reverse reaction of preparation of TBHQ. Thus, the reaction could be promoted by water. It could be shown by dealkylation of DTBHQ with conversion of 47.85% in water catalyzed by a weak acid such as acetic acid at 220℃,while no reaction occurred in xylene. In xylene, too much water would separate the catalyst acid from the reaction by dissolving the acid and cumbered the reaction. In water, the reaction temperature should be at least higher than the melting point of DTBHQ to improve the solubility of DTBHQ in water and reacting in two phase. So the reaction in water is in accordance with that in xylene.
4 CONCLUSION
The proper reaction condition for preparation of TBHQ from DTBHQ was that 20g of DTBHQ
was refluxed in 100ml of xylene for 2 hours catalyzed by 1.5g of phosphotungstic acid. The
better yield of TBHQ was 74.0% with DTBHQ conversion of 43.1%.
REFERENCE
[1]Wang W X, Li J, Li F F. Chin.Oil and Fat (Zhongguo Youzhi), 1999,24(5):59.
[2]Xia Y Z. Speciality Petrochemicals (Jingxi Shiyou Huagong), 2002,3:8.
[3] Ayabe Y, Kawahara M, Maeda M. JP,08176042,1996.
[4]Tang Y, Zhang Y M, Yang J. Chemical Journal on Internet ,2004,,6(5):33.
[5]Shin R. Mukai., Toru Sugiyama, Hajime
Tamon. Applied Catalysis A: General 256 (2003):99.
[6]Du Z X, He Y G, Min E Z. Acta Petrolei Sinica (Shiyou
Xuebao), 1999,15(1):65.
[7] He Y G, Li F, Wang P, Du Z X. Petroleum Processing and Petrochemicals (Shiyou Lianzhi
Yu Huagong), 1996,27(8):12.
[8]Gong Y F,Zhao C X,Jiang X K.Chem. J of
Chin. Univ.(Gaodeng Huaxue Xuebao),1994,15(5):685
从DTBHQ合成抗氧化剂TBHQ
(暨南大学化学系,广东 广州 510632)
摘要 通过讨论磷钨酸用量、反应时间等条件对从2,5-二叔丁基对苯二酚(DTBHQ)合成2-叔丁基对苯二酚(TBHQ)的影响,获得了较好的反应条件为1.5g磷钼酸催化20.0gDTBHQ在100ml二甲苯中回流2h,TBHQ收率74.0%,DTBHQ转化率43.1%。讨论了干燥的磷钨酸,以及在干燥的磷钨酸中先加入水混合和后加入水反应等条件下的催化反应效果,并因此获得结晶水通过影响磷钨酸的酸性大小而影响反应效果的结论, 讨论了DTBHQ脱烷基化的机理。
关键词:2-叔丁基对苯二酚;2,5-二叔丁基对苯二酚;磷钨酸;