http://www.chemistrymag.org/cji/2005/073027ne.htm |
Mar.22, 2005 Vol.7 No.3 P.27 Copyright |
(Department of Chemistry, Jinan University,Guangzhou,510632, China)
Received on
Jan.26, 2005; Supported by the Natural Science Foundation of Guangdong Province (No. 31884 and No.010369), and the National Science Foundation of China (No. 20271022). Abstract According to the "guide to the expression of uncertainty in measurement (ISO: 1993E)", the quality of result is characterized quantitatively through the analysis of procedure in determining selenocystine (SeCys) in samples by differential pulse voltammetry (DPV). The result shows the concentration of SeCys in selenium-enriched tea and selenium-enriched yeast is 887.5±199 mg/g (22.4%) and 0.64±0.103 mg/g (16.1%), respectively. The repeatability uncertainty contributes mostly to total uncertainty comparing with other uncertainty.Keywords Selenocystine, uncertainty, differential pulse voltammetry, selenium-gold film
1 INTRODUCTION
Many important decisions are based on the results of chemical quantitative analysis, so
the quality of the results is very important. This has led the use of "official
methods" to fulfill legislative and trading
requirements. The "Guide to the Expression of Uncertainty in Measurement"
was publicized by ISO [1]. The guide established general
rules for evaluation and expressing uncertainty in measurement across a broad spectrum of
measurements. Most of previous uncertainty researches were atomic absorption spectrometry
(AAS)[2] and liquid chromatography (LC) [3]. Recently, a procedure to calculate the
uncertainty associated to a binary response, of the type yes/no, originated from an
instrumental screening system was described by Pulido and his coworkers, and its
application in differential pulse anodic stripping voltammetry (DPASV) screening system to
analyse tap water, specifically, for determining Pb2+ and Cd2+ above
the UE legislation limits [4]. In this study, uncertainty in determination of
selenocystine(SeCys) by differential pulse voltammetry (DPV) using selenium-gold film
modified glassy carbon electrode ((Se-Au)/GC electrode) is evaluated.
2. EXPERIMENTAL
2.1 Apparatus and reagents
Differential pulse voltammetry (DPV) measurements were conducted on a BAS-100B
electrochemical system (BAS Co., USA).
Selenocystine (SeCys) was commercially obtained from Sigma Company. The
standard solutions of selenoamino acids were prepared in 1 mol·L-1 HCl,
and stored at 4 oC. All other chemicals were of analytical grade. The water
used has been redistilled.
2.2 Preparation of selenium-gold film modified glassy carbon electrode ((Se-Au)/GC
electrode)
The glassy carbon electrode was firstly polished using polishing cloth and 0.05mm alumina slurry and then cleaned with redistilled water for use. The selenium-gold film modified electrode was prepared by depositing selenium and gold in 8.3×10-3 mol·L-1 SeO2, 8.9×10-4 mol·L-1 AuCl3 and 0.10 mol·L-1 KCl solution at -850 mV for 60 seconds [5]. The prepared electrode ((Se-Au)/GC electrode) was cleaned thoroughly with redistilled water for use.
2.3 Samples and sample treatment
Selenium-enriched yeast (Jiangmen Centre for Biotechnological Development, Guangdong, China) was marked as containing 1023mgSe/g. Normal tea was obtained from Lianyuan, Hunan, China and selenium-enriched tea from Weishan, Hunan, China.
For the determination of free selenoamino acids by DPV in samples, prior acids hydrolysis of proteins must be employed. The method is as follows: 20.0g selenium-enriched tea and 2.00g selenium-enriched yeast were weighed respectively, then hydrolyzed in 4 mol/L HCl at 50 oC for 50 hours with N2 saturated. The hydrolyzed solutions obtained after filtering were transferred into volumetric flasks and diluted with doubly distilled water to volume for use.
2.4 Differential pulse voltammetry (DPV)
The prepared (Se-Au)/GC electrode was immersed in 0.10 mol/L KNO3 (pH=3.20) electrolyte solution containing selenocystine, and the cell was completed with an Ag/AgCl reference electrode and a platinum wire counter-electrode. After deoxygenating 10min, with N2 saturated, the reduction peak current of selenocystine was determined by DPV at a scan rate of 50mV/s and pulse amplitude of 50mV. Quantitative analysis was achieved by the standard addition method.
3. RESULTS AND DISCUSSION
3.1 Analysis of uncertainty sources
As it can be seen from the Fig. 1, the uncertainty sources mainly include concentration,
volume and mass of the solution and repeatability of sample treatment and electrode.
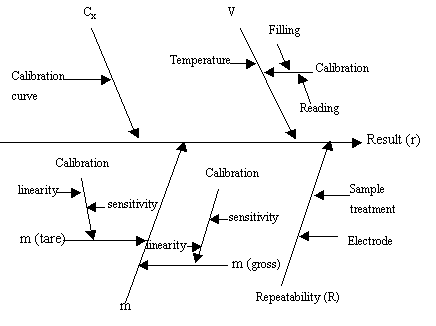
Fig. 1 Initial cause and effect diagram
3.2 Quantification of the uncertainty
sources
3.2.1 SeCys concentration Cx
For standard addition method, u (Cx) is given by
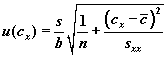 (1),
(1),
with the residual standard deviation s given by 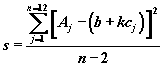 (2),
(2),
and ![]() (3).
(3).
where
b :slope
n : number of measurement for the calibration
Cx : determined SeCys concentration of the solution
![]() : mean value of the different calibration
standards (n number of measurements)
: mean value of the different calibration
standards (n number of measurements)
j : index for the number of measurements to obtain the calibration curve
For selenium-enriched yeast, the uncertainty of SeCys concentration is ![]() ; for selenium-enriched tea, the uncertainty of
SeCys concentration is
; for selenium-enriched tea, the uncertainty of
SeCys concentration is ![]() .
.
3.2.2 Volume V (V1, V2,
V3)
Calibration: The volume is calibrated according to the manufacturer's specification within
the range of ±0.06mL and ±0.04mL for 50mL graduated cylinder and for 10mL transfer
pipet, respectively. The standard uncertainty is obtained assuming a triangular
distribution. The uncertainties of volume calibration are given by ![]() ,
, ![]() .
.
Temperature: The temperature of the experiment has to be 20±2 oC.
This temperature range leads to an uncertainty in the determined volume, due to a
considerable larger volume expansion of the liquid compared with the vessel. The
coefficient of volume expansion for water is a=2.1×10-4 oC-1.
The standard uncertainty of volumes of 50mL and 10mL, assuming a rectangular temperature
distribution, is
![]() ,
, ![]() .
.
The two contributions are combined to give the standard uncertainty u
(V) of the volume V:![]() (4).
(4).
For selenium-enriched yeast or tea, the uncertainty of 10 mL transfer
pipet (V1) is given by ![]() ; two 50
mL graduated cylinders were used for stored solution (V2) and determination
solution (V3), respectively, and the uncertainty is given by
; two 50
mL graduated cylinders were used for stored solution (V2) and determination
solution (V3), respectively, and the uncertainty is given by ![]() .
.
3.2.3 Mass m
Because of the combined repeatability term identified above, there is no need to take into
account the weighing repeatability.
The weighing procedure is a weight by difference. There are two
branches, one branch is for the determination of the tare (mtare) and the other
branch is for the gross weight (mgross).
Sensitivity: If the weighing is done on the same scale and over a small
range of weight then the sensitivity contribution can be neglected.
Linearity: The calibration certificate of the balance quotes ±0.2mg
for the linearity. The balance manufactures own uncertainty evaluation recommends the use
of a rectangular distribution to convert the linearity contribution to a standard
uncertainty.
Readability: The readability of analytical balance is 0.1mg.
These give, for the standard uncertainty u (m) of the mass m, a value
of
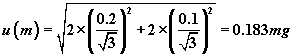 .
.
3.2.4 Repeatability R
The uncertainty for repeatability (U(R)) can be expressed as a relative standard deviation
(RSD). u(R) is given by ![]() (5),
(5),
where u(Re) is the uncertainty for repeatability of electrode expressed as RSD
(4.19% in table 1) when using three modified electrodes prepared at different times for
determination the same sample, and u(Rs) is the uncertainty in sample treatment
expressed as RSD when using the same electrode for determination samples prepared at
different times or by different persons. u(Rs) presented in Table 2 in
determinations selenium-enriched yeast and tea are 9.15%, 5.63% respectively. So the
uncertainty for electrode repeatability is smaller compared with the repeatability
uncertainty in sample treatment. The results of u(R) in determinations selenium-enriched
yeast and tea are shown in Table 3.
Table 1 The repeatability of the Se-Au/GC electrode
The electrode |
No.1 |
No.2 |
No.3 |
Average value (mA) |
RSD(%) |
Peak current (ip/ mA) |
1.19 |
1.15 |
1.25 |
1.20 |
4.19 |
Table 2 The repeatability of sample treatment
The sample |
SeCys content( mgSeCys/g) |
Average( mgSeCys/g) |
RSD (%) |
|
Selenium-enriched yeast |
No.1 |
869 |
863 |
9.15 |
No.2 |
936 |
|||
No.3 |
784 |
|||
Selenium-enriched tea |
No.1 |
0.70 |
0.64 |
5.63 |
No.2 |
0.65 |
|||
No.3 |
0.79 |
|||
No.4 |
0.54 |
|||
No.5 |
0.60 |
|||
3.2.5 Combined standard uncertainty
u(r)
The concentration of SeCys in samples is
given by ![]() (6).
(6).
In order to calculate the combined standard uncertainty of a
multiplicative expression (as above) the standard uncertainties of each component are as
follows:
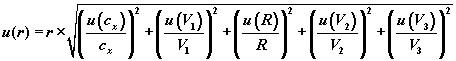 (7).
(7).
3.2.6 Expanded uncertainty U
To increase the confidence level , the
uncertainty is expressed by the expanded uncertainty (U), which is given by U =u (r)×k = u (r)×2. k is a coverage factor and when k is 2, confidence level
is 95%.
Table 3 Uncertainty for SeCys analysis
Selenium-enriched yeast |
Selenium-enriched tea |
||||||
Cx(mol/L) |
2.125×10-5 |
u(Cx)(mol/L) |
6.280×10-7 |
Cx (mol/L) |
1.524×10-7 |
u(Cx)(mol/L) |
2.270×10-14 |
V1 (mL) |
10.00 |
u(V1) |
0.016 |
V1 (mL) |
10 .00 |
u(V1) |
0.016 |
V2 (mL) |
50.00 |
u(V2) |
0.027 |
V2 (mL) |
50.00 |
u(V2) |
0.027 |
V3 (mL) |
50.00 |
u(V3) |
0.027 |
V3 (mL) |
50.00 |
u(V3) |
0.027 |
m (g) |
2.000 |
u(m) |
0.183 |
m (g) |
20.00 |
u(m) |
0.183 |
R |
1.00 |
u(R) |
0.107 |
R |
1.00 |
u(R) |
0.079 |
r ( mg/g) |
887.5 |
u( r) |
99.5 |
r ( mg/g) |
0.64 |
u(r) |
0.051 |
U |
199 |
U |
0.103 |
||||
Expression |
887.5±199 (22.4%) | Expression |
0.64±0.103(16.1%) | ||||
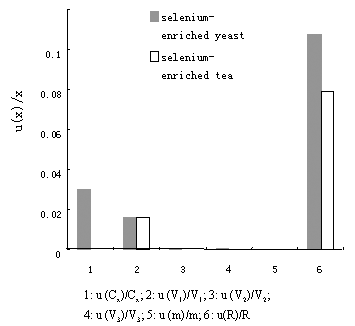
Fig.2 Uncertainty contribution of SeCys in selenium-enriched yeast and tea
All the values of the
uncertainty components are summarized in Table 3. The comparison of the uncertainty
contribution in the histogram highlights that the repeatability uncertainty is the largest
contribution to the total uncertainty. Compared to other sophisticated methods, such as
AAS and LC whose uncertainty are in general 19.0% and 24.0% respectively, herein we
reported the uncertainty in determining the concentration of SeCys in samples by DPV is
16.1-22.4%. Consequently, the result is reliable.
REFERENCES
[1] ISO 1993E. Guide to the Expression of Uncertainty in Measurement.1993.
[2] Laborda F, Medrano J,
Castillo J R. Spectrochimica Acta Part B, 2004, 59: 857.
[3] Kurfurst U, Rehnert A, Muntau H. Spectrochimica Acta Part
B, 1996, 51: 229.
[4] Pulido A, Ruisánchez I, Boqué R, et al. Analytica Chimica Acta 2002,455:
267.
[5] Bai Y, Li R, Cheng T. Analytical laboratory (China), 2003, 11: 9.
微分脉冲伏安法测定硒代胱氨酸的不确定度的评定
白燕 颜晓丽 李蕊 郑文杰
(暨南大学化学系 中国广州
510632)
国家自然科学基金(20271022)和广东省自然科学基金(31884,
010369)资助
摘要 根据国际“测量不确定度表示导则”的要求,通过对微分脉冲伏安法(DPV)测定样品中硒代胱氨酸过程的不确定度分析,对DPV测定结果的质量进行了定量表征。结果显示富硒酵母和富硒茶叶中硒代胱氨酸的含量分别为887.5±199
关键词 硒代胱氨酸; 不确定度; 微分脉冲伏安法; 硒金膜