http://www.chemistrymag.org/cji/2005/074029le.htm |
Apr. 2, 2005
Vol.7 No.4 P.29 Copyright |
Thermal thiono-thiolo rearrangement of S-allyl thiolophosphates on SiO2
Yan Zhankai, Liu Zhaojie#, Chen Weibing#(Department of Chemistry and Environmental Engineering, Yangtze University, Jingzhou, 434025; # Institute of Organic synthesis, Central China Normal University, Wuhan, 430070) Abstract The thermal rearrangement of O-allyl thionophosphates to provide S-allyl thiolophosphates was studied. The tests showed that by SiO2 O-allyl thionophosphates was heated under nitrogen atmosphere at 110 oC for 1.5 hrs, were easily converted to its thiol isomer in quantitative yields. According to this synthetic method, the twelve new compounds were synthesized and identified by IR, 1H NMR, 31P NMR and elemental analysis.
Keywords thiolophosphate; synthesis; thermal rearrangement
It is an effective
preparation method of allyl thiolophosphates by the rearrangement of allyl
thionophosphates to provide its thiol isomers. In the early years, Pudovik[1]
reported the thermal rearrangement of allyl phosphorothionates, in acetonitrile or xylene,
the reaction was very slow and in some cases gave only low conversion. Yamada[2]
first reported palladium catalyzed thiono-thiolo rearrangement of allyl thionophosphates,
they found that in the absence of the catalyst, the following reaction did not occur even
after prolonged reaction time. In this work, we have found that by SiO2
O-allyl thionophosphates 1 could easily converted to its thiol isomers
2 in quantitative yields.
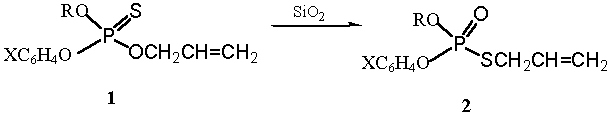
The analysis of the reaction product by TLC
indicated that only one new spot was formed. As it was confirmed by 1H-NMR,
31P-NMR, IR and element analysis, the new spot
indicated allyl thiolophosphates 2[3].
To search for the better temperature condition, we chose the starting
material 1 to be heated for 2 hours at 90 oC, 100 oC, 110 oC, 120 oC, 130 oC, respectively. The results are summarized in Table 1. The results
showed that the yield was higher at 110 oC.
Table 1 the effect of temperature in yield
temp. / oC |
90 |
100 |
110 |
120 |
130 |
isolated yield / % |
48 |
86 |
98 |
91 |
87 |
We chose 110 oC and heated the starting material 1 for 0.5 h, 1.0 h, 1.5 h, 2.0 h, 2.5 h, 3.0 h, respectively. The results are summarized in Table 2. The results showed that the reaction come to an end in 1.5 h.
Table 2 The effect of time in yieldtime / h |
0.5 |
1.0 |
1.5 |
2.0 |
2.5 |
3.0 |
isolated yield / % |
62 |
87 |
100 |
98 |
95 |
93 |
Then by choosing 110 oC and fixing the reaction time 1.5 h, the
reaction was investigated by changing substituents on aryl ring, and the results are
summarized in Table 3.
Table 3 Thermal thiono-thiolo rearrangement of
allyl thionophosphates
compds |
R |
X |
Temp. / oC |
Time / h |
Isolated yield of 2 / % |
2a |
Me |
2-Cl |
110 |
1.5 |
> 98 |
2b |
Me |
4-Cl |
110 |
1.5 |
> 98 |
2c |
Me |
2, 4-dichloro |
110 |
1.5 |
> 98 |
2d |
Me |
3, 4-dichloro |
110 |
1.5 |
> 98 |
2e |
Me |
4-SMe |
110 |
1.5 |
> 98 |
2f |
Me |
4-t-Bu |
110 |
1.5 |
> 98 |
2g |
Et |
2-Cl |
110 |
1.5 |
> 98 |
2h |
Et |
4-Cl |
110 |
1.5 |
> 98 |
2i |
Et |
2, 4-dichloro |
110 |
1.5 |
> 98 |
2j |
Et |
3, 4-dichloro |
110 |
1.5 |
> 98 |
2k |
Et |
4-SMe |
110 |
1.5 |
> 98 |
2l |
Et |
4-t-Bu |
110 |
1.5 |
> 98 |
Through the yield of
products (2a-2l) in Table 3, it revealed that the rate of reaction is
generally fast and quantitative, independent on the substitution pattern of aryl group.
The general procedure is as follows: the mixture of 1 (0.01 mol) and SiO2 (20
g) was heated under nitrogen atmosphere at 110 oC for 1.5 hrs, poured into ether, filtrated SiO2.
After evaporation of ether and purification of the residue by TLC
(silica gel, CH3CO2C2H5
-petroleum ether), pure 2 as a viscous yellow liquid was obtained.
This is the first report on the thermal [3, 3]-sigmatopic
type rearrangement of O-allyl thionophosphates on SiO2.
The full details of the reaction will be published later on.
Acknowledgement We are grateful to the Hubei Natural Science
Foundation for financial support.
[1] Pudovik A N, Aladzheva I M Zh. Obshch. Khim., 1960, 30, 2617.
[2] (a) Tamaru Y, Yoshida Z I, Yamada Y et al. J. Org. Chem., 1983, 48, 1293.
(b) Yamada Y, Suzudamo G, Yoshioka H et al. Tetrahedron Letter., 1984, 25, 3599.
[3] All new compounds (2a~2l) exhibit IR, 1H-NMR, 31P-NMR spectra and element analysis in agreement with the structure indicated. As examples, we report herein the analytical data of 2d, 2f, 2j, 2l.
2d: 1H NMR ( CDCl3, 200MHz ): d 3.57 (q, J = 5 Hz, 2H ), 3.94 ( d, J = 7 Hz, 3H ), 5.13-5.96 ( m, 3H ), 7.20-7.56 ( m, 4H ); 31P NMR (CDCl3 ): d 26.744; IR ( cm-1 ): 1638, 1578, 1475, 1407, 1272, 1099, 1033; Anal . calcd for C10H11Cl2O3PS: C 38.36, H 3.54, Cl 22.64; found C 38.41, H 3.56, Cl 22.62.
2f: 1H NMR ( CDCl3, 200MHz ): d 1.17 ( s, 9H ), 3.52 (q, J = 5 Hz, 2H ), 3.87 ( d, J = 7 Hz, 3H ), 5.13-5.84 ( m, 3H ), 7.16-7.34 ( AA'BB', J = 9 Hz, 4H ); 31P NMR (CDCl3 ): δ 26.262; IR ( cm-1 ): 1638, 1462, 1407, 1211, 1109, 1013; Anal . calcd for C15H23O3PS: C 57.31, H 7.37; found C 57.30, H 7.35.
2j: 1H NMR ( CDCl3, 200MHz ): d 1.42 ( t, 3H ), 3.57 (q, J = 5 Hz, 2H ), 4.29-4.52 ( m, 2H ), 5.11-5.96 ( m, 3H ), 7.18-7.52 ( m, 4H ); 31P NMR (CDCl3 ): d 24.903; IR ( cm-1 ): 1638, 1581, 1451, 1253, 1099, 1056; Anal . calcd for C11H13Cl2O3PS: C 40.38, H 4.01, Cl 21.67; found C 40.39, H 4.04, Cl 21.66.
2l: 1H NMR ( CDCl3, 200MHz ): d 1.30( s, 9H ), 1.40 ( t, 3H ), 3.52 (q, J = 5 Hz, 2H ), 4.14-4.36 ( m, 2H ), 5.09-5.96 ( m, 3H ), 7.15-7.34 ( AA'BB', J = 9 Hz, 4H ); 31P NMR (CDCl3 ): d 24.410; IR ( cm-1 ): 1637, 1509, 1476, 1464, 1210, 1033, 1014; Anal . calcd for C16H25O3PS: C 58.52, H 7.67; found C 58.57, H 7.68.
S-烯丙基硫逐磷酸酯在SiO2上的硫逐-硫赶热重排
严赞开,刘钊杰#,陈卫兵#
(长江大学化学与环境工程学院,荆州 434025; #华中师范大学有机合成研究所,武汉 430070)
摘要 本文探讨了O-烯丙基硫逐磷酸酯热重排制备S-烯丙基硫赶磷酸酯的反应条件。结果表明: O-烯丙基硫逐磷酸酯与SiO2混匀, 在氮气保护下, 置于110 ℃保温1.5 h, 反应可定量地完成。运用这一合成方法制备了12个不对称结构的新型硫赶磷酸酯, 所有的化合物均经IR、1H NMR、31P NMR及元素分析表征。
关键词 硫赶磷酸酯; 合成; 热重排