http://www.chemistrymag.org/cji/2005/074031pe.htm |
Apr.28, 2005 Vol.7 No.4 P.31 Copyright |
Study on the retention behavior of aromatic sulfonated compounds in the reversed-phase ion-pair chromatography with tetramethylene -oxide as organic modifier
Pang Xiuyan, Sun Hanwen, Feng Yongqiang, Liu Xiulan, Shen Shigang(Key laboratory of analytical science and technology of Hebei province, College of Chemistry and Environmental Science, Hebei University, Baoding, 071002,China) Received Mar. 17, 2005; Supported by the Natural Science Foundation of Hebei province(China, No:203110) and the Doctor Foundation of Hebei province Education Office (China, B2004402) Abstract The retention behaviors of aromatic sulfonates are discussed based on the reversed-phase ion-pair chromatography. The response of relative retention factor to the concentration of ammonium tetrabutyl bromide, tetramethylene-oxide permitted to better fit the data and to build reliable predictive models respectively. The relationship between the content of tetramethylene-oxide and the polarity, the surface tension of the mobile phase has been investigated with fluorescence probe and maximum bubble method, and the results testify that tetramethylene-oxide can obviously decreasing the surface tension and polarity. Thus it results the reduction of the sulfonates' retention in the chromatographic system. The influence of pH of the mobile phase on the retention was also discussed.
Keywords The reversed ion-pair chromatography, sulfonates, tetramethylene-oxide, retention behavior, polarity, surface tension 1 INTRODUCTION
Aromatic sulfonates such as benzene sulfonates and naphthalenesulfonates are widely used as intermediates. The excellent hydrophilic properties and low biodegradability makes them potentially hazardous [1]. Thus the detection of sulfonates becomes popular [2,3]. Many analytical techniques have been proposed for determining these compounds in water. Gas chromatography analysis needs a previous step to convert them into volatile derivates [4]. When capillary electrophoresis is used, it gives better result particularly using laser induced fluorescence detection [5]. The preferred technique, however, is still the reversed phase liquid chromatography (RP-LC) combined with UV [6], fluorescence[7] or MS[8,9],for it can offer a higher separation efficiency than that by capillary electrophoresis[3].
In the RP-LC analysis, the main factors, which influence the retention of analytes, are pH of the mobile phase and concentration of organic modifiers. Nikitas et al. studied the retention mechanism of the solute molecules from aqueous with the addition of organic modifier to the mobile phase [10-12], and the combined effect of pH and organic modifier concentration on the retention were described with six equations [12]. An attractive analysis method of ionic samples is the technique commonly referred to as ion-pair liquid chromatography (IPLC). In the RP-IPLC, pH of the eluant is adjusted in order to encourage ionization [13], and the eluent pH is recommended to be above the analytes' pKa value, in order to make sure dissociation of the acidic groups and strong ion-pair formation. Meanwhile an increasing counter-ion concentration further intensifies the retention behavior [14]. Electrolyte concentration (inorganic modifier) further influences the retention, and it decreases with the increasing electrolyte concentration [15].
In the RP-IPLC analysis of aromatic sulfonates, Leon et al. reported the detection of the C4 - C13 benzene-sulfonic acids [16], though gradient solvents were used, the retention time of C13 benzene-sulfonic acid was still near 50min. Under the condition of 10%-50% methanol-water gradient solvent, 7mM TBA and pH 6.5, Gimeno et al. had detected 14 aromatic sulfonates[17]. The retention time of 4-aminobenzenesulfonate, benzenesulfonate and 3-nitro-benzenesulfonate were 10min, 38min and 52min respectively. It is necessary to establish fast separation technique to improve the detection sensitivity and accuracy especially in the quantitative and qualitative analysis according to the peak height, area and retention time. Robert et al. had replaceed the 250 mm long column with a "fast and short" column (75×4 mm I.D., 4mm particle diameter), with the retention is reduced in half [6].
The purpose of this paper is, based upon the former works [18,19], to give further study on the retention behavior of aromatic sulfonates with THF together with methanol as organic modifier, and search for the influence of tetramethylene-oxide THF on polarity and surface tension of the mobile phase, in order to provide reliable theoretical bases for the rapid detection of sulfonates.
In this research, the polarity and surface tension of the mobile phase has been investigated with fluorescence probe and maximum bubble method [20, 21], respectively. The research provides important evidence for the relationship of the retention behavior and THF concentration. 2. EXPERIMENTAL
2.1 Reagents
Ammonium tetrabutyl bromide(analytical grade), tetramethylene-oxide (analytical grade, fresh distillated before use), methanol (HPLC quality). The following sulfonated compounds were analytical reagent and purchased from Beijing: 4-aminobenzenesulfonate, benzenesulfonate, 2-naphthol-6, 8-disulfonate, 2- naphthol-3, 6- disulfonate, 3-nitro-benzenesulfonate, dibenzal-4-sulfonate, methyl orange. All the other chemicals were commercial analytical reagent. Redistilled water was used for preparing the mobile phase.
For the experiments, a stored water solution of 1g· L-1 of each compound was prepared. Standard solutions of each compound in the concentration of 0.001-0.1 g· L-1 were prepared with the mobile phase as diluent, respectively. The pH value of the mobile phase was adjusted with K2HPO4 or KH2PO4.
2.2 Instrument
The chromatographic condition was the same as that mentioned in the former work [19]. The pH of the mobile phase was detected with PHS-3C acidometer. The fluorescence intensity of chrysene was detected under the condition of lex/lem= 365/428nm in the solution of water-methonal-THF with RF- 540 (Shimadzu, Japan), and the surface tension of the mobile phase was mensurated with simple experimental equipment.
The retention behavior of sulfonates in 15:85 methanol-water (v/v), and 30:70 methanol-water (v/v) mobile phases containing different content of THF was detected, respectively.
The influence of THF content on polarity and surface tension of the mobile phase was studied.
3 RESULTS AND DISSCUSSION
3.1 The influence of THF on the retention of sulfonates
THF could more efficiently ameliorate retention time of components than methanol [18,
22], meanwhile, it possessed low viscosity and weak UV absorption under the detected
wavelength. Figure 1 described the relationship between the sulfonates'
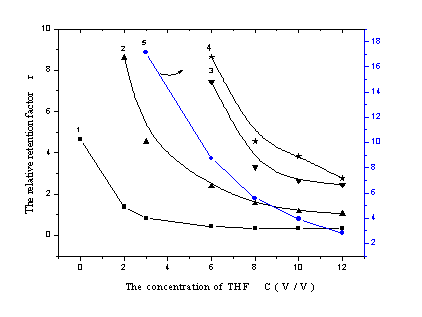
a
b Figure 1 The influence of THF concentration on sulfonates' relative retention factor
a …the 15:85 methanol - water eluent containing different percent of THF, b …the 30:70 methanol- water eluent containing different percent of THF
(1) 4-aminobenzenesulfonate (2) benzenesulfonate (3) 2-naphthol -6,8-disulfonate (4) 2- naphthol-3,6- disulfonate (5) 3-nitro-benzenesulfonate (6) dibenzal-4-sulfonate (7) methyl orange Table 1 The retention models of sulfonates under the influence of THF
| Components | Models* |
|
4-aminobenzenesulfonate |
Ln r =1.46-0.676C |
Ln r =0.293-0.87C |
benzenesulfonate |
Ln r =3.42-0.730C |
Ln r =1.82-0.559C |
2-naphthol-6,8-disulfonate |
Ln r =2.46-0.162C |
Ln r =2.84-0.368C |
2- naphthol-3,6- disulfonate |
Ln r =3.84-0.62C |
Ln r =3.06-0.405C |
3-nitro-benzenesulfonate |
Ln r =3.53-0.253C |
Ln r =2.48-0.440C |
dibenzal-4-sulfonate |
Ln r =3.87-0.255C |
|
methyl orange |
Ln r =4.84-0.185C |
|
r …the relative retention factor of sulfonates, C …the volume percent of THF in the mobile phase.
Table 2 The content of THF causing relative retention factor r a fall of 50%*
Components |
CTHF content (mL/100mL water-methanol) |
|
4-aminobenzenesulfonate |
2 (comparing with the content 0) |
2 (comparing with the content 0) |
benzenesulfonate |
3 (comparing with the content 0) |
2 (comparing with the content 0) |
2-naphthol-6,8-disulfonate |
8 (comparing with the content 6) |
6 (comparing with the content 2) |
2- naphthol-3,6- disulfonate |
8 (comparing with the content 6) |
6 (comparing with the content 2) |
3-nitro-benzenesulfonate |
6 (comparing with the content 3) |
2(comparing with 0) |
dibenzal-4-sulfonate |
12(comparing with the content 10) |
|
methyl orange |
10 (comparing with the content 6) |
|
Organic modifier could shorten retention time and ameliorate separation selectivity through reducing the polarity of the mobile phase and abating the column capacity [24]. To testify the influence of THF on the polarity of the eluent, the fluorescence probe method with chrysene as fluorescence reagent was employed. The fluorescence intensity of chrysene was detected under the condition of lex/lem= 365/428nm with water, acetonitrile, methanol, THF, acetone,hexane as solvent, respectively. The results of 42.1, 34.8, 34.6, 25.4, 26.8 and 17.5 illustrated that the emission strength of chrysene increased with the polarity of the solvents. When the solvent, which had an increasing volume ratio of THF to methanol-water, was used, a gradually depressed intensity was gained (showed in Figure 2.) The results indicated that THF could efficiently reduce the polarity of the eluent, which resulted the apparent reduction of sulfonates'
retention time.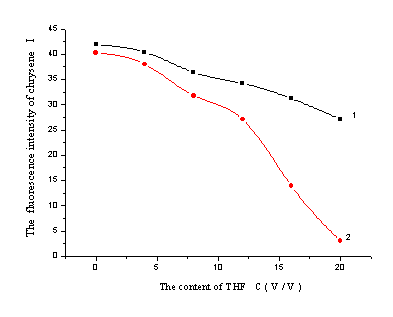
Figure 2 The fluorescence intensity of chrysene in different volume ratio of THF -methanol-water solution
1…15:85 methanol-water, 2…30:70 methanol-water
C(%)…the volume percent of THF in the mobile phase. According to Bidlingmeyer [25], there was higher surface tension between stationary phase and mobile phase. The solvent, which could modify surface tension, could also adjust the retention behavior of analytes. To study the influence of THF, the surface tension of methanol-water-THF solution was detected with the maximum bubble method. The detected solution had an increasing volume ratio of THF to methanol-water. The results were showed in Figure 3. In 15:85 methanol-water solution, the addition of 20% THF made a 16% reduction in the surface tension. While in 30:70 methanol-water solution, an 18% reduction was gained. The results declared that THF could evidently ameliorate the surface tension, and it could adjust the retention behavior of the components.
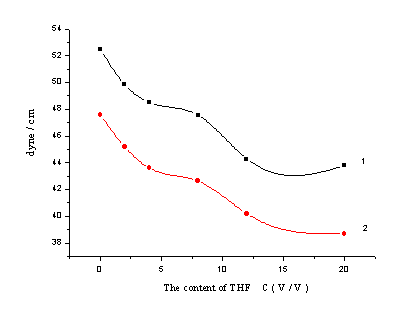
Figure 3 The influence of THF on surface tension
(1)... 15:85 methanol - water, (2)...30:70 methanol - water
C(%)... the volume percent of THF in the mobile phase.
3.2 The influence of TBA on retention
behavior
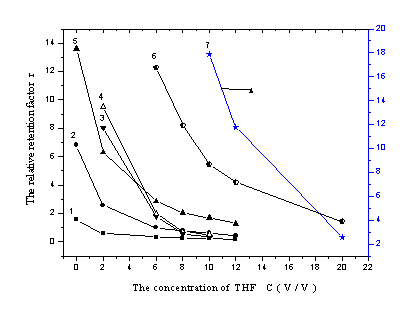
In the analysis of ion-samples, the addition of counter-ion was necessary. Wang
studied the influence of TBA on six organic acids[14], but the reaction
character of sulfonates with TBA as ion-pair reagent had not been studied systemically. In
the experiment, the effect of TBA content on the retention of the seven analytes was
showed in Figure 4. With the increasing of TBA concentration, a shortened retention time
was gained. When the TBA content was lower than the maximum column capacity, there was a
linear relationship between the Ln r and LnCTBA. The relation was
described in Table 3, and Zou reported the similar results in the studying of sulfonic
acid [26]. But different analyte had different effectual column capacity,
3-nitro-benzenesulfonate, dibenzal-4-sulfonate and methyl orange had lower value than the
others.
3.3 Influence of pH on retention feature
When the ion-sample was separated by RP-IPLC, pH of the eluant is adjusted in order to
encourage ionization [13, 14]. The effect of pH value on sulfonates' retention was determined and showed as Figure 5. The accrescent pH
value caused sulfonates' a minished retention time.
The effect of pH value on benzenesulfonate was very slight, while the change of orange was
more notable.
3.3 The separation of aromatic sulfonates
In the separation of sulfonates, the following chromatographic conditions were
employed: methanol - water (30:70, v/v)-THF (5%)-TBA (3 mmol/L), pH 6.5 with an isocratic
flow rate of 1.0 mLmin-1. The chromatograms were recorded as Figure 6, and the
retention time of 4-aminobenzenesulfonate, benzenesulfonate,
2-naphthol-6,8-disulfonate,2- naphthol-3,
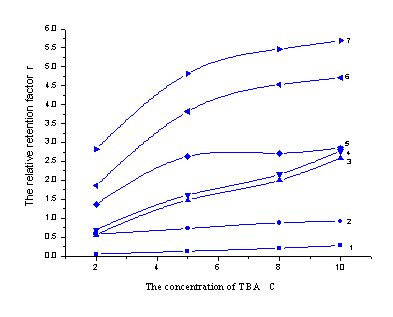
Figure 4. The influence of TBA concentration on sulfonates' retention behavior
(The mobile phase was methanol- water (30:70, v/v)- pH 6.5 and 5%THF for compound 1-5, 16% THF for compound 6-7)
(1) 4-aminobenzenesulfonate (2) benzenesulfonate (3) 2-naphthol-6,8-disulfonate (4) 2- naphthol-3,6- disulfonate (5) 3-nitro-benzenesulfonate (6) dibenzal-4-sulfonate (7) methyl orange
Table 3 The relationship between relative retention factor and TBA Concentration
Components |
Regression equation |
Relative coefficient |
Linearity
range |
4-aminobenzenesulfonate |
Lnr=-3.634+1.010LnC |
0.9989 |
~10 |
benzenesulfonate |
Lnr=-0.758+0.293LnC |
0.9949 |
~10 |
2-naphthol-6,8-disulfonate |
Lnr=-1.175+0.924LnC |
0.9962 |
~10 |
2- naphthol-3,6- disulfonate |
Lnr=-0.923+0.839LnC |
0.9975 |
~10 |
3-nitro-benzenesulfonate |
Lnr=0.0617+0.462LnC |
0.9479 |
~5 |
dibenzal-4-sulfonate |
Lnr=0.263+0.594LnC |
0.9781 |
~5 |
methyl orange |
Lnr=0.772+0.444LnC |
0.9805 |
~5 |
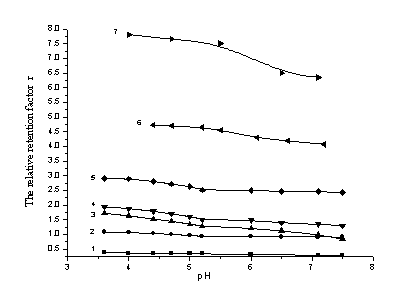
(The mobile phase was methanol- water (30:70, v/v)- TBA (3mmol/L) and 5 % THF for compound 1-5, 16 % THF for compound 6~7)
(1) 4-aminobenzenesulfonate (2) benzenesulfonate (3) 2-naphthol-6,8-disulfonate (4) 2- naphthol-3,6- disulfonate (5) 3-nitro-benzenesulfonate (6) dibenzal-4-sulfonate (7) methyl orange
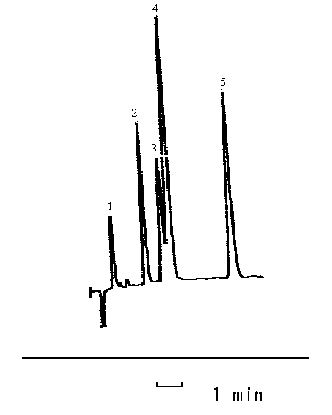
Figure 6 The chromatograms of (1) 4-aminobenzenesulfonate,
(2) benzenesulfonate,
(3) 2-naphthol-6, 8-disulfonate, (4) 2- naphthol-3,6- disulfonate
and (5) 3-nitro-benzenesulfonate
(The mobile phase was methanol- water (30:70, v/v)-THF (5%)-TBA (3 mmol/L), pH 6.5.)
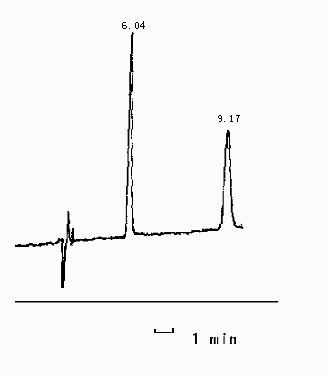
Figure 7 The chromatograms of (1)dibenzal-4-sulfonate and (2)methyl orange
(The mobile phase was methanol- water (30:70, v/v)-THF (16%)-TBA (3 mmol/L), pH 6.5.)
THF could effectively shorten the retention time through the decrease of the polarity and surface tension of the mobile phase. The amelioration was more notable than methanol. Meanwhile, the concentration of TBA and the pH of the mobile phase played more important roles in adjusting the retention behavior of sulfonates. For the ionic compounds, the research offered important proof in speedy, accuracy, qualitative and quantitative analysis.
Acknowledgements We express thanks to the Natural Science Foundation of Hebei province (China, 203110), and meanwhile, thanks for the much support of the Doctor Foundation of Hebei province Education Office (China, B2004402).
REFERENCE
[1] Lars Elsgaard, Giulio Pojana, Tommaso Miraval, Jorgen Eriksen, Antonio Marcomini.
Chemosphere, 2003, 50: 929.
[2] Socher G, Nussbaum R, Rissler K, et al. Chromatographia, 2001, 54 (1/2) : 65.
[3] Thorsten Reemtsma. Journal of Chromatography A, 2003, 1000: 477.
[4] Reemtsma T J. Chromatogr. A, 1996, 733: 473.
[5] Cugat M J, Borrull F, Calull M. Chromatographia, 1999, 50: 229.
[6] Robert Loos, Mari Carmen Alonso, Damia Barcelo. Journal of Chromatography A, 2000,
890: 225.
[7] Alonso M C, Castillo M, Barcelo D. Anal. Chem. Avta, 1999, 400:211.
[8] Thomas Storm , Thorsten Reemtsma, Martin Jekel. Journal of Chromatography A, 1999,
854: 175.
[9] Robert Loos, Mari Carmen Alonso, Damia`Barcelo. Journal of Chromatography A, 2000,
890:225.
[10] Nikitas P, Pappa-Louisi A, Agrafiotou P. Journal of Chromatography A, 2002, 946: 9.
[11] Nikitas P, Pappa-Louisi A, Agrafiotou P. Journal of Chromatography A, 2002, 946: 33.
[12] Nikitas P, Pappa-Louisi A. Journal of Chromatography A, 2002, 971:47.
[13] Bear G R. J Chromatogr, 1986, 371:387.
[14] Shu-Ping Wang, Chiou-Shyi Liao. Journal of Chromatography A, 2004, 1051: 213.
[15] Jandera P, Churacek J, Taraba B. J Chromatogr, 1983, 262: 121.
[16] Leon V M, Gomez-Parra A, Gonzalez-Mazo. Fresenius Anal. Chem, 2001,371:479.
[17] Gimeno R A, Marce R M, Rissler K, et al. Chromatographia, 2001, 53 (1/2): 22.
[18] Pang X Y, Sun H W, Wang Y H. Chromatographia, 2003, 57 (7/8) : 543.
[19] Pang X Y, Sun H W, Shen S G. Chemical Journal on Internet, 2004, 6 (2) : 15.
[20] Ning G H, Lu R J, Fang Y, et al. Chemical journal of Chinese Universities,
2002,21(8): 1196.
[21] Chen W J, Li G Z, Chai J L, et al. Acta Chimica Sinica, 2002, 60 (4): 669.
[22] Pang X Y, Sun H W, Shen S G. Chemical Journal on Internet, 2003, 5 (1) : p2.
[23] Zou H F, Zhang Y K, Lu P Z, et al. The High Performance Chromatography Method.
Analytical Chemistry series, 3 (3), Beijing: Science publishing house,2001, 209-226.
[24] Mou S F and Liu K N, Ion-chromatography Method and Application, Bingjing:
Chemical industry publishing house, 2000, 148.
[25] Bidlingmeyer B A, Deming S M, Price W P, et al. Chromatographic, 1979, 186 :
419.
[26] Zou H F, Zhang Y K, Hong M F, et al. Chinese Chromatography, 1994, 12 (4):
231.
以四氢呋喃为有机改进剂的芳香组磺酸盐的反相离子对色谱保留行为研究
庞秀言 孙汉文 冯永强 刘秀兰 申世刚
(河北省分析科学与技术重点实验室,河北大学化学与科学学院,保定,071002,中国)
摘要 本文研究了芳香族磺酸盐的反相离子对色谱保留行为,建立了磺酸盐的相对保留因子与流动相中四丁基溴化铵、四氢呋喃浓度之间的数学模型;用荧光探针法和最大气泡法考察了四氢呋喃含量对流动相的极性和表面张力的影响。结果表明:四氢呋喃可以有效的降低流动相的极性和表面张力,从而降低磺酸盐在反相离子对色谱中的保留时间。同时考察了流动项的pH对保留行为的影响。
关键词 反相离子对色谱 磺酸盐 四氢呋喃 保留行为