http://www.chemistrymag.org/cji/2005/074033pe.htm |
Apr.28, 2005 Vol.7 No.4 P.33 Copyright |
Xu
Jianzhong, Li.Yan, He Yongwu, Tian Chunming
(College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Abstract The flame retardant wool
was obtained by treating pure wool with metal oxide sol-gel system. The flame retardance
and thermal stability of it were studied by thermal analysis, FTIR, SEM, limited oxygen
index (LOI) and char yield methods. The results showed that with metal oxide composed on
the wool fiber surface, it changed the process of thermal degradation and improved
the flame retardance of the wool.
Keywords Wool, Sol-gel system, Flame retardant, LOI, TG
1. INTRODUCTION
As well as other fibers, the flame retardant study of wool has got attention and
development in the past several decades. There were many systems used to get flame
retardant of wool such as THPS, THPOH, TBPA, K2ZrF6, K2TiF6,
TiCl4 et al and some of them have been applied to industry[1-4]. But
the vital disadvantage of these systems is that they contain halogen,organic phosphor and other deleterious materials. That may do harm
to human environment.
In recent years, with the environment consciousness improving,
developing the environment friendly new flame retardant wool system (no halogen, nontoxic
) have got more attention. As far as we know, however, the study of the wool treated with
metal-oxide sol-gel system has few been reported[5-6]. In this paper, four
metal oxide sol-gel systems were chosen as flame retardant agents to improve the flame
retardant of pure wool. The thermal degradation of wool with and without flame-resistant
treatment was then studied by thermal analysis, infrared spectroscopy, scanning electronic
microscope (SEM), LOI and char yield methods.
2. EXIPERIMENT
2.1 Material
A fabric of SANLI (Hebei Province Baoding Fabric Plant, China) wool was boiled with
distilled water for 1.5 hours at 373K and dried in air at room temperature.
The dried fabric were pretreated by oxidizer[7] (
penetrating agent(Triton X-100), 0.5%; 30%H2O2 5g/L; 5%NaCO3
PH10-11; bath ratio 1:30; 95-100℃ 10min), the
negative charge of wool surface were increased. Using sol-gel system[8-10]
(metal oxide, eg: TiO2, ZrO2, Al2O3, MnO2
etc.) of positive charge steep for 3 hours. After the treatment, the fabric was
washed three times in distilled water, dried in air at room temperature and conditioned.
2.2
Thermal analysis
The differential thermal analysis (DTA) and thermogravimetry (TG) were carried out on
a DT-40 analyzer(Shimadzu, Japan)under the following conditions: sample mass: 4mg,heating
rate:10 K min-1 in static air. Calcined alumina was taken as the reference
material.
2.3
LOI value
The LOI value was measured on Stanton Redcroft FTA Instrument according to the method
described in the Chinese Standard Method GB 2406-80.
2.4
Infrared spectrometry
Infrared spectra were recorded on a Vector 27 (Bruker) spectrometer using the KBr
pellet technique.
2.5
Char yield
Char yield values were calculated by the equation of:
Char yield = w2/w1 ×100%
Where w1 and w2 are the weight of the samples before
combustion and the residue after combustion of it, respectively. This experiment was
carried out in a muffle furnace under N2 at 400℃ for 40 minutes.
3. RESULTS AND DISCUSSION
3.1 The
thermal properties of the samples
Table 1 The LOI and char yield of wool with and without flame-retardant treatment
Sample |
LOI |
Char yield/w% |
I |
24.5 |
27.47 |
II |
27 |
34.4 |
III |
39 |
15.94 |
IV |
39 |
8.4 |
V |
40.5 |
20.71 |
VI |
28 |
13.19 |
Sample I was pure wool, sample (II-VI) were pure wool respectively treated by H2O2,TiO2,
ZrO2, Al2O3, MnO2.
From Table 1,it can be seen that the LOI values of wool treated with
metal oxide sol-gel system are more than those of pure wool. It increased from 24.5 to 39. These data suggest that the
combustibility of the treated wool is reduced. The wool treated with H2O2,
LOI increased and is difficult to ignite, but produce a great deal of smoke that
will be deadly to humans when the fire took place. The wool treated with metal oxide
sol-gel system get obviously improved in flame retardant and smoke suppression.
As to the char yield, there was different case that the values of char
yield for the wool treated with metal oxide sol-gel system was lower than those of pure
wool, which is not in accordance with the traditional view that the higher the char yield,
the better the retardant capability. The sample treated with metal oxide sol-gel system,
which greatly improved retardant capability, but decreased the char yield even to 8.4%. It
shows that there would be a great change of thermal degradation process of the treated
wool compared with that of the pure wool.
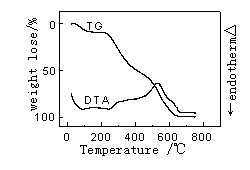
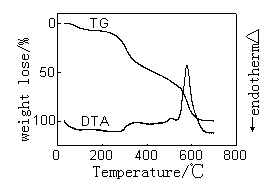
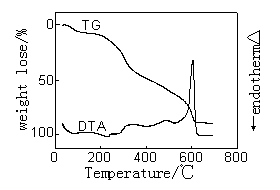
3.2 The DTA-TG curves of the samples
 |
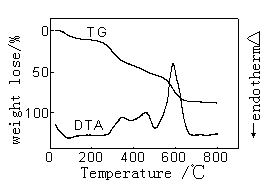 |
| Figure 1 The DTA-TG curves of pure wool | Figure 2 The DTA-TG curves of wool treated with TiO2 |
 |
 |
| Figure 3.The DTA-TG curves of wool treated with ZrO2 | Figure 4.The DTA-TG curves of wool treated with Al2O3 |
Figure 1-4 gives the
DTA-TG curves in air of the treated and untreated wools. The figures indicate that there
were three stages taking place in the wool pyrolysis process. The first, which ends at
around 120℃-160℃, is an endothermic stage and corresponds with the desorption of
water physically bound to fiber and the dehydration of wool[11].
The second important stage coincides with the temperature range over
which a number of defined pyrolysis reactions take place in wool. The hydrogen-bond
peptide helical structure ruptures and the ordered regions of the wool undergo a solid to
liquid phase change, also cleavage of the disulphide bonds occurs and a number of
volatiles are released including hydrogen sulfide and sulfur dioxide.
The third stage is an exothermic reaction that the char oxidation
reactions dominated. In Figure2-4, the exotherm in DTA curves of sample 2-4 is much
sharper and take place at higher temperature than the corresponding exotherm of pure wool
which is much broader and more prolonged. And TG curve of sample 2-4 in this process is
sharper than that of pure wool. Because of the presence of the flame retardants it appears
to have resulted in a cross-linked complex, which can be achieved in graphite-like
structure, and possibly aromatic char structure which has a high-than-expected resistance
to oxidation.
3.3 The IR spectrum of the samples
(a)
(b)
(c)
(d)
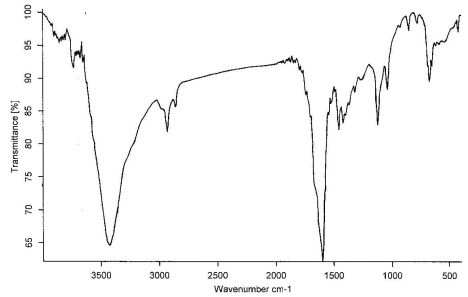
(e)
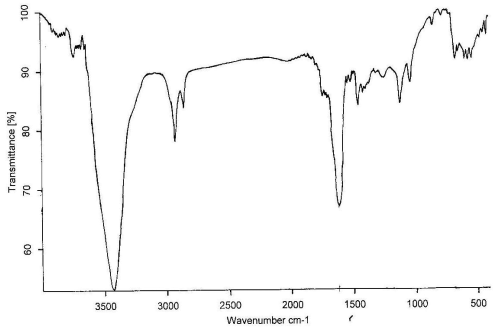
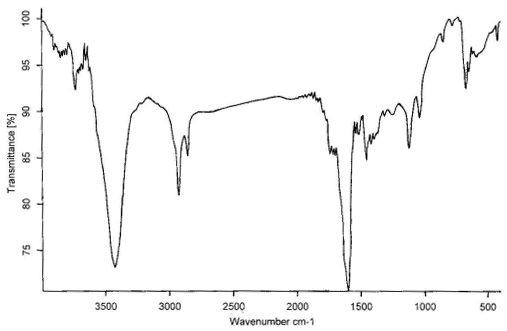
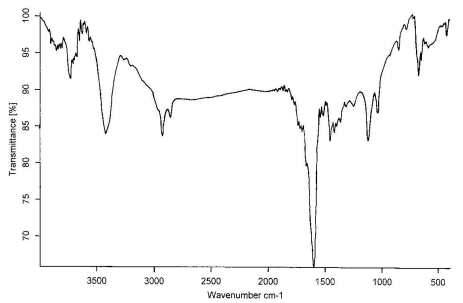
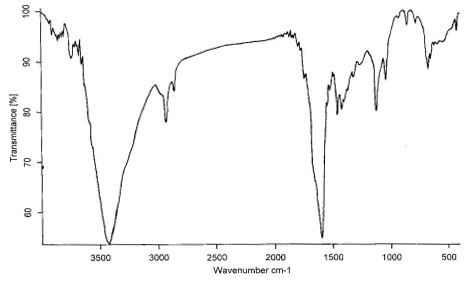
Figure 5 The IR spectrum of wool treated with ZrO2 at 200, 250,
300, 350, 400℃, respectively
The IR spectrum at 200, 250, 300, 350, 400℃
for treated wool with ZrO2 in air are shown in
Figure 5,a-e, respectively. Comparing these figures, we can see obviously the formation of
-C=C- structure at 1600 cm-1, which is very effective to heat
insulation. The absorption peaks at 3400cm-1 is the O-H flex liberation. It
moved to the lower position by the effect of the hydrogen bond. The C-O appeared at 1115
cm-1 and 1020 cm-1, however, the absorption intensity is weak and
not be obvious change.
The N-H bending frequency at 1520 cm-1 shows a reduction in
intensity, as would be expected with increasing loss of ammonia from the char. The CH2-
and CH3- stretching at 2950 and 2924 cm-1 do not show much reduction
in intensity until 400℃, indicating that the
evolution of volatile hydrocarbons commences only at higher temperature[12].
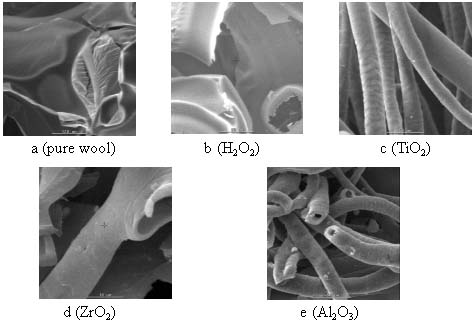
Figure 6 the SEM photo of samples
The sample (a) was pure wool, sample (b-e) were respectively treated
by H2O2,TiO2, ZrO2, Al2O3.
3.4 The SEM photo of the samples
It can be shown from Figure 6 that the char yield of the pure wool present layer
state, while the char yield of wool treated with metal oxide sol-gel system take on hollow
pipe structure inside and the fiber structure is quite distinct. It maybe the medulla
layer of fiber were destroyed, but the cuticular layer get protected due to the existence
of the oxide film on the fiber surface and form the special char yield structure. This
indicated that metal oxide can act in the condensed phase promoting char formation on the
surface, which acts as a barrier to inhibit gaseous products from diffusing to the flame
and to shield the fiber surface from heat and air.
4 CONCLUSION
The pure wool treated with metal oxide sol-gel system get flame retardant. The flame
retardant and thermal degradation of wool treated with flame retardants were studied in
air by thermal analysis, IR, SEM, limiting oxygen index (LOI) and char yield. The wool
treated with metal oxide sol-gel system, there were increasing in limiting oxygen index,
decreasing in char yield, forms hollow char yield structure of fine heat insulation. That
is favorable to improve flame retardant capability.
REFERENCE
[1]A.Richard Horrocks.Polymer, 1996, 37
(15): 3197.
[2]Baljinder K.Kandola,Samuel
Horrocks.Thermochimica Acta,1997, 294: 113.
[3]K.Fukatsu. Polymer Degradation and Stability, 2002, 75: 479.
[4]Hsiang-In Tang,Ray-Kun Lin,Tun-Fun Way et al. Polymer Degradation and Stability, 1996,
54: 373.
[5]X.Ju et al.Journal of Membrane Science, 2000, 166: 41.
[6]Ray L.Frost and Sandra M.Dutt.Journal of Colloid and Interface Science. 1998, 198: 330.
[7]Wang X Y.Zhao Z N.Qian S H.Textile Dyeing and Finishing Journal (Ranzheng
Jishu),1997,19: (1): 19.
[8]Yin L S.Journal of Functional Materials (Gongneng Cailiao),1999,30 (4): 407.
[9]Chen D R.Journal of Applied Chemistry (Yingyong Huaxue),1996,13(4): 80.
[10]Yao N.Xiong G X.Sheng S S.et al.Journal of Fuel Chemistry and Technology(Ranliao
Huaxuexuebao), 2001,8 (29):80.
[11] Xu J Z. Gao M. Guo H Z. et al. J.Fire Science, 2002, 20 (3): 227.
[12] Tian C.M., Shi Z.H., Zhang H.Y.,et al.Thermochimica Acta 1996, 284 (2):435.
金属氧化物溶胶体系对羊毛的阻燃处理及其热特性研究
徐建中* 李妍
何勇武 田春明
(河北大学化学与环境科学学院,河北,保定,071002)
摘要
用金属氧化物溶胶体系对羊毛纤维进行阻燃处理,所得的阻燃样品用热分析,剩碳率,氧指数,红外光谱,扫描电镜等分析方法对其热降解行为进行了研究。根据阻燃羊毛的热降解行为的变化,对金属氧化物溶胶体系的阻燃机理做了初步的探讨。结果表明:经过金属氧化物处理的羊毛纤维,起始热降解温度比未处理的下降,氧指数升高,剩碳率降低,阻燃性增强。
关键词 金属氧化物;溶胶;羊毛;阻燃