http://www.chemistrymag.org/cji/2005/075036pe.htm |
May 20, 2005 Vol.7 No.5 P.36 Copyright |
Synthesis and crystal structure of amine bis(phenolate) ytterbium potassium bimetallic complex
Zhang Yong, Ma Mengtao, Yao Yingming, Shen Qi
(Key Laboratory of Organic Synthesis of Jiangsu Province, Department of Chemistry and
Chemical Engineering, Suzhou University, Suzhou 215006, China)
Received on Mar. 12, 2005; Supported by National Natural Science Foundation of China (No. 20472063) and the Key Laboratory of Organic Synthesis of Jiangsu Province.
Abstract Reaction
of anhydrous YbCl3 with two equivalents of LK2 in tetrahydrofuran,
after crystallization from toluene in the presence of DME, afforded a six-coordinate amine
bis(phenolate) ytterbium potassium bimetallic complex, L2YbK(DME)2
[L = Me2NCH2CH2N{CH2-(2-O-C6H2-But2-3,5)}2,
DME = dimethoxyethane]. The central ytterbium atom is coordinated by four oxygen
atoms and two nitrogen atoms from two amine bis(phenolate) ligands, resulting in a
distorted octahedron. The potassium atom is coordinated by two oxygen atoms of the amine
bis(phenolate) ligands and four oxygen atoms of two DME molecules in a distorted
octahedron.
Keywords Ytterbium, Amine bis(phenolate) ligand, Crystal structure, Synthesis.
1 INTRODUCTION
In recent years, the application of bridged bis(phenolate) ligands in organolanthanide
or organolanthanoid chemistry has attracted considerable attention, since bridged
bisphenols have been found to be able to act as dianionic ligand, which has the advantage
of avoiding ligand redistribution reactions, and providing a stereochemically rigid
framework for the metal center that could affect stereospecific transformations[1-9].
Furthermore, some of these complexes have shown some interesting catalytic activity[5-9].
However, all the lanthanide complexes supported by bridged bis(phenolate) ligands reported
so far are neutral complexes, except our recent report about the synthesis and crystal
structure of lanthanide "ate" complexes
supported by the carbon-bridged bis(phenolate) ligand MBMP2- [MBMP =
2,2'-methylene-bis(6-tert-butyl-4-methyl-phenolate)][10]. In order to
elucidate the influence of the bis(phenolate) ligands on the molecular structure of the
bridged bis(phenolate) lanthanide "ate" complexes,
we used the amine bis(phenolate) group [Me2NCH2CH2N{CH2-(2-O-C6H2-tBu2-3,5)}2]
(L2-) as an ancillary ligand and synthesized the ytterbium "ate"
complex L2YbK(DME)2. The results are
reported as follows.
2 EXPERIMENTAL
2.1 General considerations
All manipulations were performed under a purified argon atmosphere using standard
Schlenk techniques. Solvents were degassed and distilled from sodium benzophenone ketyl
under argon prior to use. Ligand LH2[11] and anhydrous YbCl3[12]
were prepared according to the literature procedures. All other reagents were purchased
from Aldrich and used as received without further purification. Melting point was
determined in sealed Ar-filled capillary tube. Ytterbium analysis was carried out by
complexometric titration. The content of potassium was determined with a Hitachi 180-80
polarized Zeeman atomic absorption spectrophotometer. Carbon, hydrogen and nitrogen
analyses were preformed by direct combustion on a Carlo-Erba EA 1110 instrument. The IR
spectrum was recorded on a Magna-IR 550 spectrometer.
2.2 Synthesis of L2YbK(DME)2
A solution of LH2 (5.26 g, 10.02 mmol) in THF (20 mL) was added dropwise to
a K suspension (20.25 mmol) in THF at room temperature. The mixture was stirred
continually for 14 h, and then the pale yellow solution was added to a suspension of YbCl3
(1.40 g, 5.01 mmol) in THF (20 mL). The reaction mixture was stirred for 12 h at room
temperature, the deposition was separated by centrifugation. The solvent was removed in
vacuum and the residue was extracted with toluene and DME. Colorless crystals were
obtained from toluene solution at room temperature in 5 days. Yield: 6.10 g (84.7%). M.p.
200.2-200.6 °C (dec.). Anal. Calc. (found) for C76H128KN4O8Yb:
C 63.48 (63.35), H 8.97 (9.00), N 3.90 (4.06), Yb 12.03 (12.07), K 2.72 (2.95). IR (KBr
pellet, cm-1): 2955(s), 2901(s), 2866(s), 1605(m), 1474(s), 1439(m), 1416(m),
1358(m), 1046(m), 880(m), 833(m), 505(s).
2.3 X-ray crystallography
Intensity data for the title complex were collected on a Rigaku Mercury CCD area
detector using graphite monochromated MoKa radiation (l = 0.71070Å). Diffraction data were collected by w -2q scan technique. The total
reflections of 80931 were collected, of which independent reflections of 18239 could be
observed and used in the structure refinement. The structure was solved by direct method.
The positions of all non-hydrogen atoms were refined anisotropically with full-matrix
least-squares on F2. In the final difference map, the residuals are
3.105 and –1.303 e/Å3,
respectively.
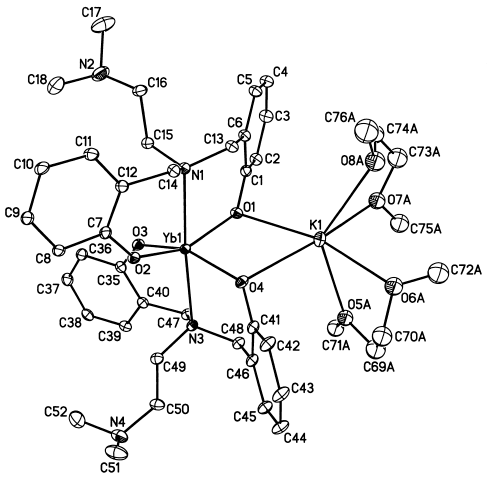
Fig. 1 Molecular structure of the title complex. Two
disordered dimethoxyethane groups are shown in one possible orientation.
3 RESULTS AND DISCUSSION
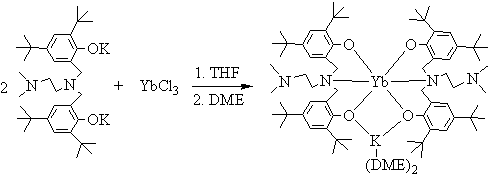
Reaction of anhydrous YbCl3 with two equivalents of LK2 (M = Li,
Na, K) in THF, after workup, produced the colorless crystals in high yield. The
composition of this complex was established as L2YbK(DME)2 by
elemental analyses (C, H, N, Yb and K). Further single crystal X-ray diffraction study
revealed that the new ytterbium “ate” complex was formed; an alkali metal atom is connected to one oxygen
atom from each of the amine bis(phenolate) ligands as shown in Scheme 1. The title complex
is well soluble in THF and toluene, but insoluble in hexane.
Scheme 1
The structure of the title compound is
shown in Fig. 1. The crystallographic data and analysis parameters are shown in
Table 1. Selected bond lengths and angles are listed in Table 2.
Table 1 Crystal data and structure analysis parameters
Complex |
L2YbK(DME)2 |
V (Å3) | 7972.6(13) |
Crystal size (mm3) |
0.42×0.40×0.35 |
Z |
4 |
Empirical formula |
C76H128KN4O8Yb |
Dc (g/cm3) |
1.198 |
Temperature (K) |
193(2) |
F (000) |
3060 |
| Wavelength (Å) | 0.71070 |
Scan mode |
w -2q |
Formula weight |
1437.96 |
q range for data collection (° ) | 3.01-27.48 |
Crystal system |
monoclinic |
Absorption coefficient (mm-1) |
1.276 |
Space group |
P21/c |
Reflections collected |
80931 |
| a (Å) | 18.5341(17) |
Independent reflections |
18239 |
| b (Å) | 15.4772(14) |
Goodness-of-fit |
1.116 |
| c (Å) | 28.696(3) |
R [I > 2s (I)] |
0.0493 |
| a (° ) | 90 |
wR2 |
0.1137 |
| b (° ) | 104.414(2) |
Maximum diff. peak (e/Å3) | 3.105 |
g (° ) |
90 |
Minimum diff. peak (e/Å3) | –1.303 |
Table 2 Selected bond lengths (Å) and angles (° )
Bond lengths |
|||
Yb(1)-O(1) |
2.183(3) |
Yb(1)-O(2) |
2.131(3) |
Yb(1)-O(3) |
2.110(3) |
Yb(1)-O(4) |
2.173(3) |
Yb(1)-N(1) |
2.514(3) |
Yb(1)-N(3) |
2.547(3) |
K(1)-O(1) |
2.765(3) |
K(1)-O(4) |
2.843(3) |
K(1)-O(5) |
2.857(7) |
K(1)-O(6) |
2.666(11) |
K(1)-O(7) |
2.838(8) |
K(1)-O(8) |
2.812(9) |
Yb(1)-K(1) |
3.9221(11) |
||
Bond angles |
|||
O(1)-Yb(1)-O(2) |
154.92(10) |
O(2)-Yb(1)-O(3) |
95.75(11) |
O(3)-Yb(1)-O(4) |
155.25(10) |
O(1)-Yb(1)-N(1) |
77.10(10) |
O(1)-Yb(1)-O(4) |
88.22(11) |
O(2)-Yb(1)-N(3) |
105.88(10) |
N(1)-Yb(1)-O(2) |
77.99(10) |
O(1)-Yb(1)-O(4) |
88.22(11) |
N(3)-Yb(1)-O(1) |
98.93(10) |
O(1)-K(1)-O(6A) |
165.9(2) |
O(1)-K(1)-O(4) |
65.44(8) |
O(5A)-K(1)-O(7A) |
81.2(2) |
O(4)-K(1)-O(5A) |
94.97(16) |
O(8A)-K(1)-O(4A) |
131.1(2) |
O(7A)-K(1)-O(8A) |
59.5(2) |
X-Ray structure analysis reveals that the title complex is a dinuclear ytterbium-potassium complex with two phenoxo bridges. The overall molecular structure is different from the case using MBMP2- as the ancillary ligand, in which the discrete ion-pair complexes are formed in the presence of DME[10]. The central metal ytterbium atom is six-coordinated by four oxygen atoms and two nitrogen atoms from two amine bis(phenolate) ligands in a slightly distorted octahedron. Unlike those in the neutral amine bis(phenolate) lanthanide complexes[6,7,9], the sidearm donor did not coordinate to the central metal. One bridging oxygen atom (O(1)), one terminal oxygen atom (O(2)) and two nitrogen atoms (N(1), N(3)) can be considered to occupy equatorial positions within the octahedron about ytterbium center with S O-Yb-N near to 360° . The oxygen atoms from another bridging phenolate ligand (O(3)) and terminal phenolate ligand (O(4)) occupy axial positions, and the corresponding bond angle is distorted away from the idealized 180° to 155.25° , presumably due to the steric demand of the bridging phenolate ligands. The Yb-O(Ar) distances range from 2.110(3) to 2.183(3) Å, giving an average of 2.149(3)Å. The averaged terminal Yb-O(Ar) bond length is 2.121(3) Å, which falls within the range previously reported for the terminal Yb-O(Ar) distance of 2.080(1)-2.295(1) Å[9]. The averaged bridging Yb-O(Ar) bond length is 0.05Å longer than the terminal one, which is comparable well with that in [Na{Nd(OC6H3-Ph2-2,6)4}] (0.04 Å)[14], but apparently shorter than the value found in (THF)La(OC6H3-iPr2-2,6)2(m-OC6H3-iPr2-2,6)2Na(THF)2 (0.108 Å)[13]. The Yb-N bond lengths are 2.514(3) and 2.547(3) Å, respectively, which are in accordance with those reported in the literatures when the difference in ionic radii is considered[6,7,9]. The bite angles O(1)-Yb-O(2) and O(3)-Yb-O(4) of 154.92(10)° and 155.25(10)° in the title complex is apparently larger than that found in (THF)2Nd(MBMP)2Na(THF)2 (95.51(8)° )[10]. The O(2)-Yb-O(3) bond angle between two terminal phenolate ligands is 95.75(11)° , which is apparently smaller than the corresponding bond angle in (THF)2Nd(MBMP)2Na(THF)2 (162.90(6)° ); whereas the O(1)-Yb-O(4) angle between two bridging phenolate ligands is smaller at 88.22(11)° , which is similar to that in (THF)2Nd(MBMP)2Na(THF)2 (79.62(5)° )[10]. These differences can be attributed to that lengthening the linker between two phenolates increases the flexibility of the bis(phenolate) ligand. Associated with the increased flexibility of the amine bis(phenolate) in this complex is the difference in the dihedral angles between the arene rings. The dihedral angles are 45.97(16)° and 46.70° for the amine bis(phenolate) ligands containing O(1)O(2) and O(3)O(4) in the title complex, respectively; while the corresponding values are 90.64(8) and 81.79(9)° in (THF)2Nd(MBMP)2Na(THF)2, respectively[10]. The potassium cation is coordinated by four oxygen atoms from two DME molecules and two oxygen atoms from two amine bis(phenolate) ligands to form a slightly distorted octahedron. Two DME molecules are disordered due to strong thermal motion.
4. CONCLUSION
In summary, we have successfully synthesized a new soluble amine bis(phenolate)
lanthanide "ate" complex, and characterized
its structural feature by X-ray crystallography. In comparison with the carbon-bridged
bis(phenolate) ligand, MBMP2-, lengthening the linker between two phenolates
increases the flexibility of the amine bis(phenolate) ligand.
REFERENCES
[1] Schaverien C J, Meijboom N, Orpen A G. J. Chem. Soc., Chem. Commun., 1992, (2):
124.
[2] Gribkov D V, Hultzsch K C, Hampel F. Chem. Eur. J., 2003, 9 (19): 4796.
[3] Arnold P L, Natrajan L S, Hall J J et al. J. Organomet. Chem., 2002, 647 (1-2): 205.
[4] Skinner M E G, Tyrell B R, Ward B D et al. J. Organomet. Chem., 2002, 647 (1-2): 145.
[5] Ma H Y, Spaniol T P, Okuda J. Dalton Trans., 2003, (24): 4770.
[6] Cai C X, Toupet L, Lehmann C W et al. J. Organomet. Chem., 2003, 683 (1): 131.
[7] Cai C X, Amgoune A, Lehmann C W et al. Chem. Commun., 2004, (3): 330.
[8] Deng M Y, Yao Y M, Shen Q et al. Dalton Trans., 2004, (6): 944.
[9] Kerton F M, Whitwood A C, Willans C E. Dalton Trans., 2004, (15): 2237.
[10] Xu X P, Ma M T, Yao Y M et al. Eur. J. Inorg. Chem., 2005, (4): 676.
[11] Tshuva E Y, Goldberg I, Kol M. Organometallics, 2001, 20 (14): 3017.
[12] Tayler M D, Carter C P. J. Inorg. Nucl. Chem., 1962, 24: 387.
[13] Clark D L, Hollis R V, Scott B L et al. Inorg. Chem., 1996, 35 (3): 667.
[14] Deacon G B, Feng T, Junk P C et al. J. Chem. Soc., Dalton Trans., 1997, (7): 1181.
胺基双芳氧基镱钾双金属配合物L2YbK(DME)2的合成与晶体结构
张勇 马猛涛 姚英明* 沈琪*
(江苏省有机合成重点实验室,苏州大学化学化工学院,苏州215006,中国)
2005年3月12日收稿;国家自然科学基金(20472063)和江苏省有机合成重点实验室资助项目
摘要 通过胺基双芳氧基钾与无水YbCl3在四氢呋喃中反应,然后在甲苯和DME的混合溶剂中重结晶,合成了双金属配合物L2YbK(DME)2
[L = Me2NCH2CH2N{CH2-(2-O-C6H2-But2-3,5)}2],并测定了其晶体结构。结构测定表明,中心金属原子Yb与胺基双芳氧基的四个氧原子和两个氮原子配位,构成一个扭曲的八面体几何构型;钾原子与胺基双芳氧基的两个氧原子和两个DME分子的四个氧原子配位,也形成一个扭曲的八面体。
关键词 镱、胺双芳氧基配体、晶体结构、合成