http://www.chemistrymag.org/cji/2005/075037pe.htm |
May 20, 2005 Vol.7 No.5 P.37 Copyright |
(aSchool of Chemical and Material Engineering, Southern Yangtze University, Wuxi, Jiangsu 214036, b Key Lab of Colloid and Interface Chemistry for State Education Ministry, Shandong University, Ji'nan 250100) Received on Mar. 16, 2005; Supported by the National Natural Science Foundation of China (No. 20473034) and the Taihu Scholar Foundation of Southern Yangtze University (2003)
Abstract Spontaneous vesicle formation
was observed from dicetyl dimethyl ammonium chloride (DCDAC) and its mixture with sodium
bis- (2-ethylhexyl) sulfosuccinate (AOT) in aqueous and aqueous/ethanol solvents. The vesicle
observation was further supported with Biology Microscope, negative-staining
transmission electric microscope (TEM) and Atomic Force Microscope (AFM). By using the
vesicle as the carrier, the entrapment efficiency and release efficiency of All-trans
Retinoic Acid (ATRA) were measured with dialysis bag-Ultraviolet Spectrophotometry method.
Results showed the entrapment efficiency of pure DCDAC solution in 50% (v/v) ethanol-water
solution with the concentration of 2.0×10-3 mol/L reached 28%, while the
solution with the same concentration and ratio of ethanol, DCDAC/AOT=1/9 reached 10%.
Keywords Dicetyl dimethyl ammonium chloride (DCDAC), Vesicle, All-trans retinoic
acid (ATRA).
1 INTRODUCTION
All-trans retinoic acid (ATRA) is one of the
efficient anti-cancer drugs for the treatment on Acute Promyelocytic Leukemia(APL), as well as some skin diseases such as folliculi pili
cornification, psoriasis, mite, and so on [1]. However, it is lipo-soluble,
poisonous and unstable, especially sensitive to light, temperature as well as atmosphere
and easily to be oxidized [2]. The desirable means to overcome these
disadvantages is to use the microcapsule technology in the manufacture of ATRA medicament.
Vesicle is a
prospective drug carrier to increase the stability of drugs and the solubility of
lipophilic substance in aqueous solution [3-4]. It also can be used to control
the release ratio of drug in bodies and consequently, to enhance the effect of treatment
and reduce the toxicity of drug [5-8].
Since the formation of the first spontaneous vesicle from didodecyl
dimethyl ammonium bromide (DDAB) by Kunitake in 1977[9], up to date, vesicles
have been obtained from diverse surfactants [10]: single-chain surfactants as
well as dichain ones; mixtures of ionic surfactants (anionic/cationic systems) as well as
of ionic with nonionic ones. As ATRA is not solvable in water but in ethanol, the
spontaneous vesicle formed in high concentration of ethanol solution will be its desirable
carrier. The structure of Dicetyl dimethyl ammonium chloride (DCDAC) is similar to DDAB [9];
therefore, it is hopeful that spontaneous vesicle can be obtained from DCDAC solution or
its mixtures. However, to our knowledge, no work has been reported on the vesicles from
DCDAC, nor about the formation of vesicles in high ethanol concentration. The present work
is, by using atomic force microscope (AFM), negative-staining transmission electric
microscope (TEM) , biologic microscope (BM) and dialysis bag-ultraviolet spectrophotonetry
as the tools, firstly to explore the formation of vesicles from single DCDAC or its
mixture with AOT in pure aqueous solution as well as in ethanol solution and secondly, to
encapsulate ATRA with the vesicles.
2 EXPERIMENTAL
DCDAC was donated by Witco Co. without any
further purification (>98%). Sodium bis- (2-ethylhexyl) sulfosuccinate (AOT) was
obtained from Fluka Co. without any further purification (>98.5%). Ethanol was A.R.
grade. Water was doubly distilled.
Vesicles were
prepared with magnetic stirrer by mixing surfactants in proper proportions and
concentrations. Formation of spontaneous vesicles was firstly judged by the special
appearance of vesicle solution, i.e., the transparent blue color [10-11] and
Biology Microscope(BM) (AQJ-2010, Zhengjiang Angel Fine Instrument Co.), then further
confirmed by TEM (H-7000, HITACHI Japan) and AFM(Veeco
Co. USA). Entrapment efficiency and release
efficiency of ATRA were measured with dialysis bag (molecular weight: 14000, half girth:
33mm, Huamei Biology Medicine Co.) and Ultraviolet Spectrophotometry (TU-1901, Beijing
Purkinje General Instrument Co.). Firstly ATRA was separately dissolved in the fixed
volume ratio of aqua/ethanol, one example was added with surfactants but the other was
not. Then the samples were separately put into dialysis bags to measure entrapment
efficiency and release efficiency by using the solution with the same volume ratio of
ethanol and quantity of ATRA as reference liquid. For each time 10 ml solution was
used and the dialysis was immersed into 100 ml reference liquid being held in an
Erlenmeyer flask and stirred at room temperature. After a certain time, 5 ml sample
was taken out from the bag to measure the entrapment efficiency and release efficiency
with Ultraviolet Spectrophotometry and another 5ml of aqua/ethanol solution was added into
the bag to make its total volume unchanged.
3 RESULTS AND DISCUSSION
3.1 Spontaneous vesicle formation from pure DCDAC solution
The most direct method to judge whether there are vesicles formed is by the special
transparent blue appearance of surfactant solution. The solution of DCDAC with a
concentration 1.0×10-3 mol/L appeared blue, which indicated there were
vesicles spontaneously formed. With the increase of the concentration of DCDAC the blue
color and turbidity of solution both got deeper, but it could be cleared again by the
addition of ethanol in the solution. Figure 1a is the TEM photograph of the DCDAC with a
concentration 1.5×10-3 mol/L in 50% ethanol.
It has been reported that many kinds of double-chain surfactant can
form vesicles spontaneously, especially those with equal lengths such as C12 alkyl chains [9]. DCDAC has double equal length alkyl
chains of C16 so that it is reasonable that it can form spontaneous vesicles in aqueous
solution, the more interesting is that it even can form vesicle spontaneously in high
concentration of ethanol solution.
3.2 Spontaneous vesicle formations from DCDAC/AOT solution
Figure 1b, 1c were the TEM and AFM photographs of the DCDAC/AOT=2/8 with total
concentration 1.0×10-3 mol/L in volume ratio of ethanol being 50% and aged for
2 days, in which vesicles were observed as spherical closed aggregation with uniform size
and the diameter was about 200 nm measured by AFM. Table 1 was the detail appearance
change of mixtures with the total surfactant concentration (1.0×10-3 mol/L) in
different proportion of ethanol. For the mixtures DCDAC/AOT=1/9 and 2/8, solution
appearance changed to clear when the ethanol volume proportion increases to 75%, which
mean vesicles disappearing at this proportion [12]. For the mixtures
DCDAC/AOT=3/7 and 7/3, though the double salt was dissolved by massive addition of
ethanol, no any vesicles was observed. But in systems DCDAC/AOT=8/2, 9/1, transparent blue
could be observed even in 75% ethanol solution.
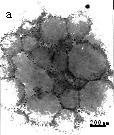


Fig.1 Photos of vesicles (a) TEM photograph of pure DCDAC solution, C=1.5×10-3 mol/L, Vethanol=50%;(b)TEM photograph DCDAC/AOT=2/8, Ctotal=1.0×10-3 mol/L,Vethanol=50%.and (c) AFM photograph of the same sample with (b) (where the arrowhead indicates vesicles.).
Table 1 Appearance changes after mixing the DCDAC and AOT solution (Ctotal=1.0×10-3 mol/L)
DCDAC/AOT |
1/9 |
2/8 |
3/7 |
4/6 |
5/5 |
6/4 |
7/3 |
8/2 |
9/1 |
0 |
Bluish |
Bluish |
Turbid |
Turbid |
Turbid |
Turbid |
Turbid |
Bluish |
Bluish |
25% |
Bluish |
Bluish |
Turbid |
Turbid |
Turbid |
Turbid |
Turbid |
Bluish |
Bluish |
50% |
Bluish |
Bluish |
Turbid |
Turbid |
Turbid |
Turbid |
Turbid |
Bluish |
Bluish |
75% |
Clear |
Clear |
Clear |
Clear |
Clear |
Clear |
Clear |
Bluish |
Bluish |
Though at
the very high proportion of ethanol, vesicle formation was very difficult (clear
appearance of solution), DCDAC/AOT mixture could form vesicle (bluish appearance of
solution) even at much high concentration of ethanol (80%) in solution as the total
surfactant concentration increase. Vesicles can be seen in the TEM photographs of vesicle
with DCDAC/AOT=1/9 and 5.0×10-3 mol/L
total surfactant concentration in 80% ethanol solution.
Generally, ethanol
can destroy remarkably the vesicle system/liposome when the concentration reaches around
6%-7% [13]. However, some vesicles systems can be stable in solution with
higher concentration of ethanol [14] or other polar solutions such as n-propyl
alcohol [15], isopropyl alcohol, and tetrahydropyrane [16]. Two
effects are caused by adding polar solutions [12]: first decreasing the
polarity of system and then inhibiting the formation of vesicle; second, decreasing the
dielectric constant of solvent and increasing the electrostatic attraction between cation
surfactant and anion surfactant. The electrostatic attraction is an advantage to the
vesicle systems. Electrostatic attraction is stronger than the lyophobic action, which is
the primary effect after addition of ethanol. So, vesicle systems can be stable in ethanol
solutions.
2.3 The capability of encapsulating ARTA with DCDAC and DCDAC/AOT vesicles
Since the ATRA is soluble in ethanol but not in water, the simple method of encapsulating
ATRA with DCDAC or DCDAC/AOT vesicles is by adding surfactants in the ethanol
solution that contains ATRA. The absorption peak of ATRA in ethanol solution is 338 nm and
it had been tested that the addition of DCDAC or DCDAC/AOT does not affect the absorption
position. Therefore, this peak could be used to measure the entrapment efficiency of ATRA.
Samples of ATRA vesicle system appeared yellow in the dialysis bag when freshly prepared,
then this color got light with aging due to the escape of free ATRA from the bag, and at
last, the concentration of free ATRA out/in side of the bag reached to the balance after a
certain time. So the release efficiency fr and the entrapment efficiency
fe of ATRA could be measured by the absorbency at the balance
point of concentration with the following equations:
![]() (1)
(1)
![]() (2)
(2)
Where W0
was the quantity of ATRA outside of the dialysis bag and Wt was the
total ATRA in solution.
The release efficiency of the samples of DCDAC and DCDAC/AOT=1/9 with
the total surfactant concentration 2.0×10-3 mol/L and total ATRA 0.1g/L (3.3×10-4
mol/L) in 50% volume ratio of ethanol had been measured with aging. About 4 days later,
the dialysis reached the concentration balance point. The corresponding entrapment
efficiency is 10% for the mixture but much higher (28%) for the single DCDAC component
with the same surfactant concentration at this point. The reason may be that the bilayer
structure of DCDAC/AOT mixture was much tighter and as the result, ATRA molecules were
hard to get into the vesicles and leave a large quantity of ATRA as free in the solution.
4. CONCLUSION
Spontaneous vesicles could be formed
either in single component of DCDAC or its mixture with AOT both in aqueous and
aqueous/ethanol solvents. Deposit could be transformed to vesicles in ethanol/aqueous
solution at high surfactant concentration. Entrapment efficiency of ATRA in the single
DCDAC component solution was higher than that in its mixture with AOT.
REFERENCES
[1] Huang M E, Ye Y C, Chen S R, et al.
Blood, 1988, 72: 567.
[2] Hwang S R, Lim S J, Park J S, et
al. Int. J. Pharm., 2004, 276: 175.
[3] Chen W J, Zhai L M, Meng X G, et al. Chinese Sci Bull., 2003, 48 (13): 1338.
[4] Wang Z W, Chen W J, Liu X J, et al. Science China (Kexue Tongbao), 2005, 2: 123.
[5]Amidon G L. J. Contril. Rel., 2001, 71 (2): 213.
[6] Janout V. J. Am. Chem. Soc., 2001, 123 (40): 9926.
[7] Deborah M, Laurence T, Christine D, et al. Int.J.Pharm., 2000, 200: 73.
[8] Singer S J, Nicolson G L. Science, 1972, 175: 720.
[9] Kunitake T, Okahata Y. J. Am. Chem. Soc., 1977, 9: 3860.
[10] Chen W J, Li G Z, Zhai L M, et al. Chinese Chem Lett, 2003, 14 (3): 327.
[11] Bergmeier M, Hoffmann H, Witte F. J. Colloid. Interf. Sci., 1998, 203: 1.
[12] Huang J B, Han F, Wu T. Acta. Phys. Chim. Sin., 2003, 19 (8): 779.
[13] Simon S A, Mclntosh T. J. Biochim. Biophys. Acta., 1984, 773: 169.
[14] Huang J B, Zhu B Y, Zhao G X, et al. Langmuir, 1997, 13: 5759.
[15] Huang J B, Zhu B Y, Mao M, et al. Colliod. Polym. Sci., 1999, 227: 354.
[16] Huang J B, Yang R, Zhu B Y, et al. Colloid and Surface A, 2000, 74: 403.
DCDAC、DCDAC/AOT自发囊泡的形成及其对全反式维甲酸的包封研究
顾明艳a,王正武a*,陈文君b,
袁文轲a,王仲妮b,李干佐b
(a江南大学化学与材料工程学院,无锡,214036;b山东大学胶体与界面化学教育部重点实验室,济南,250100)
摘要
用生物显微镜、透射电镜和原子粒显微镜,研究了双十六烷基二甲基氯化铵(DCDAC)单组分,及其与二(2-乙基己基)琥珀酸钠(AOT)复配体系在水溶液及乙醇/水溶液中自发囊泡的形成。并用透析椬贤夥止夤舛确ú饬苛怂媚遗萏逑刀匀词轿姿幔?/FONT>ATRA)的包封率。结果表明,浓度为2.0×10-3
mol/L、乙醇体积分数为50%的DCDAC单组分体系对ATRA的包封率可达到28%,而相同总表面活性剂浓度和相同乙醇体积分数的DCDAC/AOT=1/9复配体系对ATRA的包封率仅为10%。
关键词 双十六烷基二甲基氯化铵,囊泡,全反式维甲酸