http://www.chemistrymag.org/cji/2005/075038pe.htm |
May 20, 2005 Vol.7 No.5 P.38 Copyright |
(1 Key Laboratory of Organic Synthesis of Jiangsu Province, School of Chemistry and Chemical Engineering Suzhou University, Jiangsu 215006; 2 Jiangsu Polytechnic University, Changzhou 213016) Received on Apr.15, 2005. This work is funded by the National Natural Science Foundation of China (No.20476066, 20076031), the Key Laboratory of Organic Synthesis of Jiangsu Province (No.S8109114) , the Project of Industrialization of high and novel technology of universities in Jiangsu Province (JH03050) and Natrural Science Foundation Of Jiangsu Province (No. BK2002042). Abstract Polyimide with p-p Conjugate main chain was prepared by polycondensation of Benzoguanamine(BGA) and Pyromellitic Dianhydride under microwave irradiation. The coordination reaction of Eu3+ with this polymer was carried out in solid state and also prompted by microwave irradiation. The polymeric complexes were characterized by Fourier transform Infrared spectra (FTIR), Fourier transform Raman spectra (FTRS) and X-ray powder diffraction. The effect of microwave irradiation in different power and irradiation time was studied. Composition of two reactants and the reaction temperature had impressive effect on the Eu content in the complexes. Introduction of Eu3+ into polymer network also led to different fluorescent emission from that of original polymer. The polymeric complexes exhibited the specific peaks of Eu3+, while the emission bands of original polyimide disappeared.
Keywords microwave irradiation; solid phase coordination; fluorescence
Microwave was first applied in organic
chemical synthesis by Gedby [1] and Giguere[2] , it was brought a
good deal of attention. Microwave has many advantages, such as heating inside, heating
quickly, heating selectively, saving energy and so on. At present microwave irradiation
has been extensively used in inorganic chemistry and organic chemistry [3]. In
the area of polymer synthesis, microwave has been used to carry out the radical
polymerization of vinyl monomers, such as 2-hydroxy ethyl methacrylate [4],
Styrene [5], MMA [6] and the curing of polymer such as Epoxy resins
and polyurethane [7] as well as the imidization of polyamic acids (PAA)[8].
During the course of studies, we have already reported the microwave irradiation addition
and condensation polymerization. [9] The study of solid phase coordination
reaction at low temperature was very few, and the coordination reaction of polymers under
microwave irradiation has not been reported.
Trivalent Rare earth based complexes, such as Eu3+ and Sm3+
complexes with beta-diketone, possessed distinctive fluorescent properties, namely,
the narrow emission bands, high quantum efficiency and long fluorescent lifetime[10].
These complexes were extensively studied due to the potential application in light
emitting materials with monochromatic emission. In this paper, polymeric complexes of Eu3+
and backbone-conjugated polyimide were synthesized by assistance of microwave irradiation.
The fluorescent properties of polyimide and polymeric complexes were studied.
1.1 Synthesis
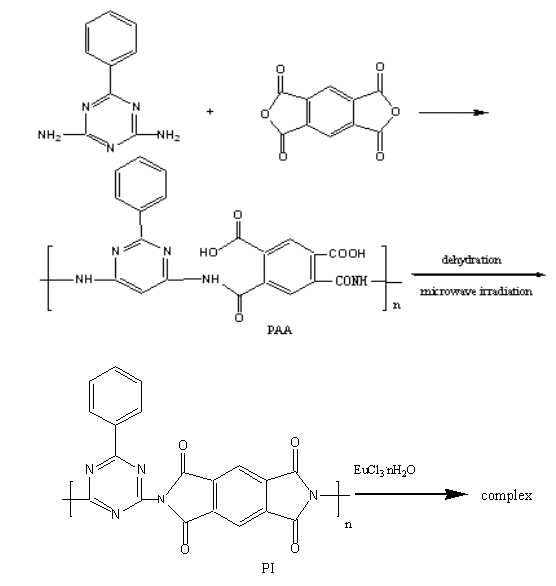
1. 1.1 BGA-PMDA Polyimide
BGA was dissolved in anhydrous DMF. PMDA was added to the solution in three portions. The temperature of polymerization (75oC) was controlled by modulating the power of the microwave oven. After 1 hour, the solution was poured into CHCl3, and the precipitate formed was washed in THF and dried under vacuum at 45oC overnight, polyamic acid (PAA) was obtained. The polyimide was synthesized through imidiztion of PAA by microwave irradiation for 10 minutes.
1. 1. 2 Europium chloride (EuCl3·nH2O)
Europium oxide was dissolved in hydrochloric acid (6mol/L), and the solution was heated to remove excessive solvent. The Europium chloride(EuCl3·nH2O) was precipitated as colorless crystal.
1. 1. 3 Preparation of complex (Eu3+-BGA-PMDA polyimide) by microwave irradiation
The polyimide and EuCl3·nH2O was mixed in the prescribed proportion and milled perfectly .The mixture was put into the domestic microwave oven and irradiated under continuous and intermittent irradiation for some time at a given temperature. The resultant mass was washed in turn with anhydrous ethanol and DMF either for several times. Then the complex was dried under vacuum and weighed.
1.1. 4 Preparation of complex (Eu3+-BGA-PMDA polyimide) by conventional heating method
The well mixed polyimide and EuCl3·nH2O were heated at the same temperature (the same temperature as the maximum temperature of the microwave radiation) for a prescribed time. Then the reaction mixture was washed and dried by the same procedure as employed in microwave irradiation methods.
1. 2 Measurements
1. 2. 1 Determination of Eu content in complex
The contents of Eu in complex were determined by a method of EDTA titration[11].
1. 2. 2 Spectra Measurements
IR spectra: Mangna-550. FT Raman Spectra: Nicolet FT-Raman 960. X-ray microanalyses: DMAX-3C. Fluorescence spectra: SPECTRAPRO 750i photometer, lex=532nm.
2 RESULTS AND DISCUSSION
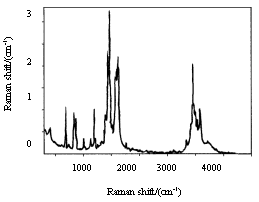
The wide peak in Fig.1 and Fig.2 is the absorption peak of –COOH in the residual of PAA. There are two characteristic peaks of
acid imide 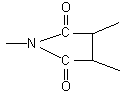 at
1787.77 cm-1 and 1849.22 cm-1 in Fig.1, while the two peaks
disappeared in Fig.2, which showed the coordination between polyimide and Eu3+.
Peak at 1650cm-1 in Fig.1, due to the carbonyl of
at
1787.77 cm-1 and 1849.22 cm-1 in Fig.1, while the two peaks
disappeared in Fig.2, which showed the coordination between polyimide and Eu3+.
Peak at 1650cm-1 in Fig.1, due to the carbonyl of ![]() , also showed the
residual of PAA, which also could be proved by the existence of peaks (3450-3291.98cm-1)
of N-H in Fig.1 and Fig.2 as well. The peaks at 1350.28-1244.24cm-1 were
corresponding to the C-N. Owing to the coordination of Eu ion,the structure of the
polyimide formed is showed as follows:
, also showed the
residual of PAA, which also could be proved by the existence of peaks (3450-3291.98cm-1)
of N-H in Fig.1 and Fig.2 as well. The peaks at 1350.28-1244.24cm-1 were
corresponding to the C-N. Owing to the coordination of Eu ion,the structure of the
polyimide formed is showed as follows:
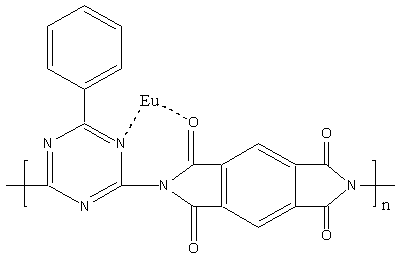
The coordination of Eu ion with O of C=O and N of N=C resulted
in the IR peak shift of C--N in polyimide from 1350.28cm-1 and 1244.24cm-1
to 1383.63cm-1 and 1325.44cm-1 respectively. We are
assured that the Eu ion could coordinate with polyimide through analysis on IR spectra of
BGA-PMDA polyimide and form complex of Eu3+--BGA-PMDA polyimide.
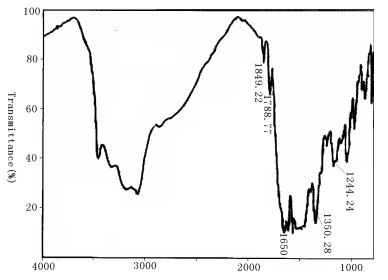
Fig. 1 IR spectrum of BGA-PMDA
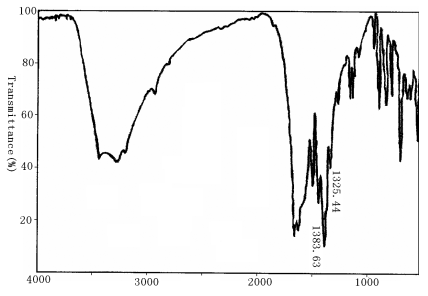
Fig. 2 IR spectrum of Eu3+-BGA-PMDA polyimide
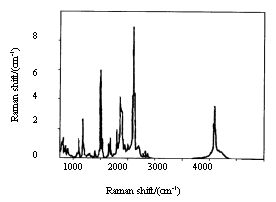
The
complex structure of Eu3+-polyimide was also investigated by measuring the FT
Raman spectra (Fig.3, 4). The polyimide has absorption bands around 1597cm-1,
1570cm-1 and 1164cm-1 corresponding to C=N, C=O and C-N. After the
coordination of Eu3+ and polyimide, these bands also shift to 1612cm-1,
1582cm-1 and 1192cm-1 respectively. These shifts also suggest the
coordination between Eu3+ ion and N or O atom [12].
 |
|
| Fig.3 FTRS of BGA-PMDA polyimide | Fig.4 FTRS of complex of Eu3+-BGA-PMDA polyimide |
X-ray diffraction
patterns of EuCl3·nH2O,
polyimide, the mixture of EuCl3·nH2O
and polyimide and the complex are different, which suggests that the complex is a new
matter, not the simple mixture of EuCl3·nH2O and polyimide.
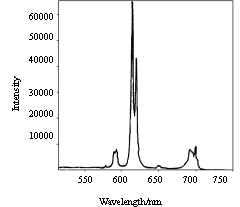
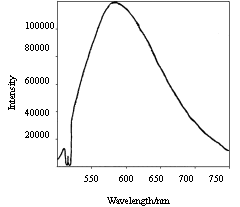
Figure 5 and Figure 6 showed emission spectra of polyimide and
complex. The spectra of polyimide have a wide peak around 600nm. But the complex behaves
fluorescent property of the Eu3+. In Figure 6, the peaks at 580nm, 590-596nm,
610-620nm, 650nm and 687-703nm are due to the 5D0→7F0,5D0→7F1,5D0→7F2,5D0→7F3 and 5D0→7F4 transition. The peak at 613nm ascribed to
5D0→7F2 is
the strongest, and the followed fluorescence intensity of this article is the intensity of
peak at 613nm. The polymeric complexes emittd red light with narrow emission bands, while
the emission bands of original polyimide could not be observed in Fig.6 due to the
effective energy transfer from polymeric ligands to Eu3+.
 |
|
| Fig.5 Fluorescent emission spectrum of BGA-PMDA polyimide | Fig.6 Fluorescent emission spectrum of complex Eu3+-BGA-PMDA polyimide |
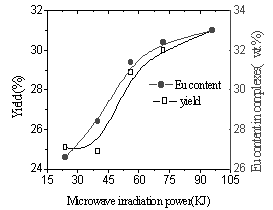
Influence of microwave irradiation power on complex
 |
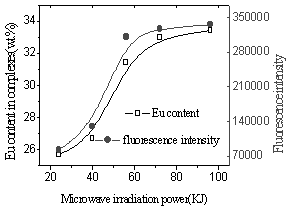 |
Fig.7 Influence of
microwave irradiation on Eu concent and yied |
Fig.8 Influence of
microwave irradiation power on Eu content and fluorescence intensity (Eu ion vs polymer structure units is 2mmol/1mmol), at 90oC) |
In Fig.7, yield of complex and content of Eu ion in the complex were found to increase with increasing microwave radiation power. The possible reason is that solid phase reaction includes four steps (diffusion, reaction, nucleation and growing) [13]. When the irradiation energy is added, the time of Eu ion and polyimide react is longer. On the other hand microwaves can cause rapid shift of the dipoles in the molecules, and this process may be considered as molecular agitation able to replace mechanical stirred [14]. So the reaction groups of the polyimide enwrapped in the tangled polymer chains have been exposed and react with Eu ion or shorten the distance between Eu ion and reaction group which is far originally to have chance to react. In Fig.8, the fluorescence intensity increased with increasing Eu content in the complexes, which suggests that Eu ion disperse uniformly in the complex [15].
Influence of proportion of Eu ion and polyimide on the complex
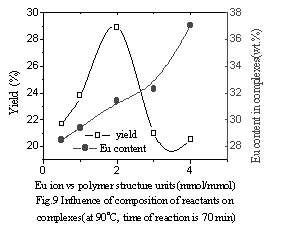 |
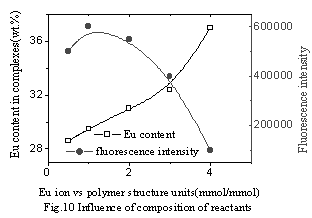 |
| Fig.9 Influence of
composition of reactants on complexes (at 90oC, time of reaction is 70 min) |
Fig.10 Influence of
composition of reactants on Eu content and fluorescence intensity (at 90oC , time of reaction is 70min) |
In Fig.9, Eu content in the complex increased with increasing Eu content in the reactants. However, the yields of the complex reached a maximum and subsequently decreased with increasing Eu content in the reactants. The possible reason is that at the beginning of the reaction, the more quantity of Eu ion added in the reactants, the more ion coordination with the polymer, intermolecular complex was mainly formed and the yield of the complex increased. But after the yield reached a maximum, intramolecular complex was mainly formed and increased quantity of Eu ion in the complex was less than increased quantity of the reactants, which leads to decreasing yields of the complex. In Fig.10, in the complex the fluorescence intensity reached a maximum at a Eu content as much as 30wt.% and subsequently decreased with increasing Eu content. It was suggested that when Eu content was less than 30wt.%, Eu ion was dispersed uniformly in the complex, and that when Eu content was more than 30wt.%, there were ionic aggregates existed in the complex, which led to fluorescence quenching.
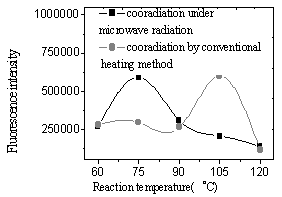
Coordination by microwave irradiation compared with conventional heating method
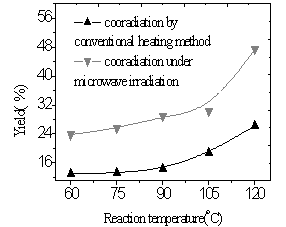 |
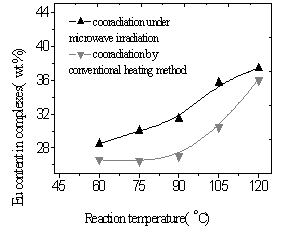 |
| Fig.11 Influence of
reaction temperature on Eu content (time of reaction is 70min, Eu ion vs polymer structure units is 2mmol/1mmol) |
Fig.12
Influence of reaction temperature on Eu content |
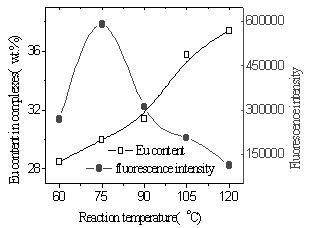 |
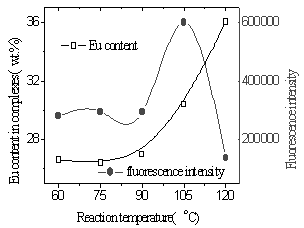 |
| Fig.13 Inflence of
reaction temperature on Eu content and fluorescence intensity under microwave radiation (time of reaction is 70 min, Eu ion vs polymer structure units is 2mmol/1mmol) |
Fig.14 Influence of
reaction temperature on Eu content and fluorescence intensity by conventional heating
method (time of reaction is 70min, Eu ion vs polymer structure units is 2mmol/2mmol) |
|
|
| Fig.15 Influence of reaction temperature on fluorescence
intensity by two methods (time of reaction is 70min, Eu ion vs polymer structure units is 2mmol/1mmol) |
From Fig.11 and 12, the yields of the complex and Eu content of the complex were found to increase with the increasing of reaction temperature of two methods. It was illustrated that higher temperature promoted reaction. Because diffusion is the slowest step of phase reaction and reaction rate is decided by diffusion rate. Rising reaction temperature is in favor of diffusion. At higher temperature especially higher than 100oC, volatilized crystal water of EuCl3·nH2O formed liquid bridge between Eu ion and polymer and lacuna of crystal lattice formed when H2O and HCl left reaction system, which were in favor of reaction. In the complex the fluorescence intensity reached a maximum at a Eu content as much as 30wt.% and subsequently decreased with increasing Eu content (Fig.13,14). It was suggested that when Eu content was lessen than 30wt.%, Eu ion was dispersed uniformly in the complex, and that when Eu content was more than 30wt.%, there were ionic aggregates existed in the complex, which led to fluorescence quenching. Fluorescence intensity reached maximum at 75oC under microwave irradiation but at 105oC using conventional heating, which was shown in Fig.15. Because transport properties and diffusion properties of molecules improved by microwave irradiation, which enhanced reaction rate. So microwave irradiation may have both thermal activation and non-thermal interaction.
REFERENCE
[1] Gedby R, Smith F, Westaway K. Tetradron Lett., 1986, 27 (3): 279.
[2] Giguere R J, Bray T L, Duncan S M. Tetrahedron Lett., 1986, 27 (41): 4945.
[3] Liao X J, Raghavan G S V, Yaylayan V A. Tetrahedron Lett., 2002, 43 (1): 450.
[4] Tang Y C, Fu Z, Liu G M. Chin J. Chem. Phys., 2003, 16 (4): 321.
[5] Zhu X L, Chen J Y, Cheng Z P. J. Appl. Polym. Sci., 2003, 89 (1): 28.
[6] Zhu X L, Chen J Y, Zhou N C. Europ. Polym. J., 2003, 39 (6): 1187.
[7] Mijovic J, Wijaya J. Polym Compos., 1990, 11 (3): 184.
[8] Lewis D A, Summers J D, Ward T C. J. Polym. Sci., Part A: Chem., 1992, 30 (8): 1647.
[9] Lu J M, Jiang Q S, Zhu X L et al. J. Appl. Polym.
Sci., 2001,79(2): 312.
[10] Baek N S, Roh S G, Kim Y H. Journal Of
Nonlinear Optical Physics & Materials, 2004, 13 (3-4): 627.
[11] Jiang Z C, Cai R X, Zhang H S. Analysis Chemistry of rare earth elements, science
publishing company, China, 2000.
[12] Meconnell A A, Brown D H, Smith W E. Spectrochim. Acta 1982, 38A (2): 265
[13] Miao Q, Xin X Q, Hu C. Chinese Journal of Chemical Physics, 1994, 7 (2): 118
[14] Stadler A, Kappe C O. European Journal Of Organic Chemistry, 2001, (5): 919.
[15] Banks E, Okamoto Y, Ueba Y. J. Appl. Polyn. Sci., 1980, 25 (3): 359.
微波辐射条件下Eu3+-- p-
p共轭缩聚配合物的合成与表征薛莹雯1 戴蔚荃1 路建美1,2*
(苏州大学化学化工学院,江苏省苏州市; 215006 ,江苏工业学院,江苏常州市 213016)
摘要 在微波辐射条件下,通过苯代三聚氰二胺和均苯四甲酸二酐的缩聚得到了主链共轭的聚酰亚胺,并在微波辐射条件下通过固相配位反应合成了Eu3+与聚酰亚胺的配合物。通过红外、拉曼及X-射线粉末衍射对产物进行了表征。探讨了微波辐射时间(功率)、反应配比、温度、Eu含量对转化率的影响。Eu3+与聚酰亚胺的配合物在荧光发射光谱上表现出Eu3+的特征发射峰。
关键词 微波辐射 固相配位 荧光