http://www.chemistrymag.org/cji/2005/075039pe.htm |
May 20, 2005 Vol.7 No.5 P.39 Copyright |
Microwave-assisted allylations in distilled water and its reaction mechanism
Xu Xiaolan, Hui Ailing, Zhou Cunliu, Zha Zhenggen, Wang Zhiyong
(Department of Chemistry, University of Science &
Technology of China, Hefei 230026)
Received Mar. 16, 2005; Supported by the National Natural
Science Foundation of China (50073021) and National Natural Science Foundation of Anhui
Province (01046301).
Abstract Microwave
Irradiation(MWI)was applied in the allylations of carbonyl compounds in water
firstly. In the presence of mediator (Zn/SnCl2) MWI can promote the allylation
greatly. Also, the allylation of carbonyl compounds with unactive allyl chloride in water
was realized under MWI. The ketones that hardly allylate with allyl halide (Br or Cl)
proceeded the allylation smoothly by using MWI to give the corresponding product in good
yield. Compared with the traditional aqueous allylation, the reaction yield was improved
greatly and the reaction time was reduced dramatically.
Keywords Aromatic aldehydes, Water, Catalysis, Halides, Allylation
Homoallylic alcohols are well known as
important building blocks in many biological active molecules such as macrolides,
polyether and antibiotics [1]. Recently a variety of aqueous
Barbier-reactions were developed to give the homoallylic alcohols. However, the reactions
normally take longer time [2] and a variety of assistant agents [3]
were often involved. Particularly, the allylations of some ketones in water
hardly occur in catalytic amount of auxiliary agents such as Lewis acids and mediators [4].
Therefore, how to promote Barbier reaction in net water becomes one of the crucial points
in the aqueous allylations. As far as we know, microwave irradiation (MWI) plays an
important role in organic reactions [5]. One
of the features from MWI is to reduce the reaction time from days or hours to minutes or
seconds. Secondly, microwave heating in the sealed reaction vessels is a highly efficient
heating technique. Besides, the heating procedure is easily controlled since the energy
input starts and stops immediately when the power is
turned on or off. So far, MWI has been employed in organic reactions such as Heck–reaction [6], Suzuki reaction [7] and
other reactions [8] to enhance the reaction efficiency, giving the excellent
results. Moreover, a few reports [9] about microwave-assisted reaction in
aqueous media were published recently. However, there is no report about
microwave-assisted allylation of carbonyl compound in water yet. In order to enhance the
allylation efficiency, MWI was employed in the allylation in our lab. As a
result, the corresponding homoallylic alcohols were obtained in high yield and the
reactions were finished in a short reaction time. Herein, we wish to report zinc-mediated
allylations of carbonyl compounds with allyl halide (Cl and Br) in distilled water via
MWI.
First of all, the microwave power and the irradiation time were
optimized. When the general microwave oven is applied in the organic reaction, the
microwave power is in the range of 100-400W and the irradiation time is several minutes.
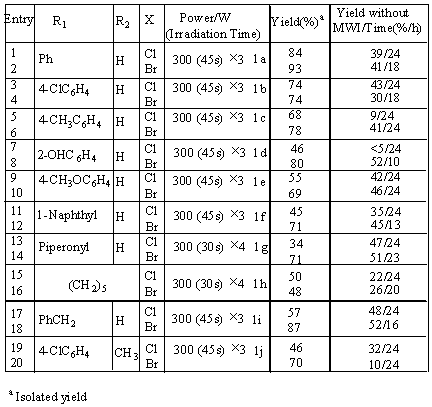
In our experiments such as the reaction of benzaldehyde with allyl bromide (Scheme 1), the
best reaction condition is 300W for the microwave power and 30-45second for the
irradiation time, while the other conditions, such as the power is 80w, 150w, 450w and the
irradiation time is 15s, 60s and 90s respectively, afford the corresponding products in a
lower yield of 30-60%. On the base of this optimization, different Lewis acids were
employed in the allylation respectively. The experimental results were listed in Table 1.
Scheme 1
![]()
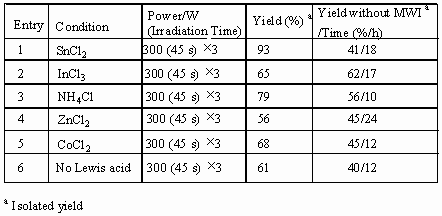
From Table 1 it was found that stannous dichloride is the most efficient to mediate the allylation under the microwave, giving the highest yield of 93% (entry1, Table 1). In comparison with the allylation without microwave, the yields were enhanced greatly and the reaction time was shortened absolutely. Under microwave condition, moreover, the dosage of SnCl2 plays a crucial role in the allylation. The optimized dosage is 0.2 equivalent of SnCl2. Neither more nor less than 0.2 equivalent of SnCl2 gives a good result.
Then the allylations of different aldehydes and ketones with allyl bromide were carried out under the optimized condition (Scheme 2). The results were listed in Table 2. From which, it was found that allylation under microwave gave higher yields and shorter reaction time than the allylations without microwave.
Scheme 2
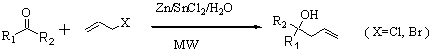
Table 2 Allylation of different carbonyl
compounds in distilled water
This result
intrigued us to substitute allyl bromide with allyl chloride in order to enlarge organic
reactions in water. The allylation by allyl chloride will be more interesting since allyl
chloride is more popular and cheaper chemical raw material. To the best of our knowledge,
however, the allylation by allyl chloride hardly occurs in water since the energy of C-Cl
bond is higher than that of C-Br bond. In fact, under the same condition, the allylation
by allyl chloride gave lower yield. Therefore the auxiliary agent is necessary to be used
to enhance the allylation by allyl chloride. Usually, phase transfer catalyst (PTC) is
effective to activate C-Cl bond [10]. After a lot of trials,
tetrabutyl ammonium bromide was chosen as a PTC to catalyze the allylation, giving the
best result.
Therefore the mediator system for the allylation by allyl chloride was
optimized as Zn (1.5mol)-SnCl2 (0.2mol)-Bu4NBr (0.1mol) under MWI.
Under this condition, the allylations of different aldehydes and ketones with allyl
chloride were carried out in water. The experimental results and the comparisons with the
conventional allylation were listed in Table 2.
From Table 2, it was found that some aldehydes such as benzaldehyde,
4-chloro-benzaldehyde and 4-methyl-benzaldehyde reacted with allyl chloride smoothly to
give the corresponding adducts in good yields, while the allylations without the MWI gave
the corresponding product in lower yields and the reactions took long time (entries 1-6,
Table 2). Particularly, the allylation of 2-hydroxyl-benzaldehyde gave the corresponding
product in good yield without tedious protection and deprotection of hydroxyl group under
the condition (entries 7 and 8, Table 2). As for 4-chloro-benzyl acetone and
cyclohexanone, the allylations gave the corresponding alcohols in very low yield and took
longer reaction time without MWI. Under MWI, however, the allylations of ketones give the
corresponding adducts in good yields and reaction time was shortened from 20-24 h to 2
minutes (entries 15, 16, 19 and 20, Table 2). In a word, the employment of the microwave
in the allylation enhanced the reaction yield greatly and shortened the reaction time
dramatically.
The mechanism of organic reaction accelerated by MWI is very
complicate. Here two factors from MWI affect the reaction. One is the heat effect [11],
which can increase the kinetic energy of the reactant molecules so that the probability of
collision among molecules is enhanced, resulting in improvement of the reaction. The other
is non-heat effect [12], which maybe plays more important role in the reaction
than the heat effect. Normally, non-heat effect affects the reaction by changing the
reaction path, resulting in the reduction of the reaction active energy. As a result, the reaction rate is enhanced dramatically and the
reaction yield is improved. Here, in this bimetal mediation system, Zn transferred the
electron to Sn2+ firstly and reduced Sn2+ to Sn (0). Then in-situ Sn
(0) inserted in the C-Br bond of the allyl bromide, resulting in the formation of allyltin
halide (1). In conventional allylation, this polar organotin compound (1)
should attack the nucleophilic center in the aldehyde to give rise to the corresponding
addition product. However, under MWI, (1) was irradiated mostly because of the
strong polarity of this molecule, leading to the formation of the allyl radical (2).
On the other hand, Zn can transfer the electron to the aldehyde to afford radical anion (3),
which can connect (2) to generate the corresponding homoallylic alcohol (4)
after capturing a proton in water. In this way, the reaction active energy was reduced
largely, as shown in scheme 3.
Scheme 3
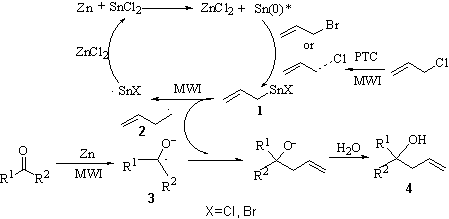
Actually, we
obtained Zn(OH)X and Sn(OH)X (X=Br, Cl) from the white precipitation and gray
precipitation respectively. Also, some hexa-1, 5-diene was observed. These experimental
facts supported the mechanism proposed here. As a result, the reaction time was reduced
dramatically and the reaction yield was increased because the reaction path was changed
from nucleophilic addition to radical polymerization. As for allyl chloride, the insertion
of tin into the C-Cl bond is difficult because of the shorter bond length and higher bond
energy of C-Cl bond in comparison with the C-Br bond. In the
presence of tertrabutyl ammonium bromide (PTC), the polarity of the C-Cl bond can be
enhanced by the action of PTC so that tin can insert this bond to afford the allyltin
halide (1). Then the reaction followed the scheme 3, realizing the allylation of
carbonyl compound with allyl chloride.
In conclusion, the microwave was applied into the allylations of
carbonyl compounds in water firstly. A novel bimetal mediation system, Zn-SnCl2-MW,
was employed in the allylations. The reaction yields were improved greatly
and the reaction time was reduced dramatically. Also, the reaction mechanism under the MWI
was studied. Experimental results indicated that the reaction path was changed from
nucleophilic addition to radical polymerization. Therefore MWI improved the allylations
via changing the reaction path. This result will intrigue us to improve the other
reactions and develop new reactions by using MWI further. The extensive application of MWI
is in progress at our laboratory.
REFERENCES
[1] Roush W R. In Comprehensive Organic Synthesis, Vol. 2; Trost B M, Fleming
L, Heathcock C H, Pergamon Press: Oxford, 1991, 1.
[2] (a) Li C J, Lu Y Q. Tetrahedron Lett., 1995, 36: 2721.
[3] (a) Petrier C, Einhorn J, Luche J L. Tetrahedron Lett., 1985, 26: 1449; (b) Loh
T P, Yin Z, Song H Y, Tan K L. Tetrahedron Lett., 2003, 44: 911.
[4] Andrews P C, Peatt A C, Raston C L. Tetrahedron Lett., 2002, 43: 7541.
[5] (a) Lidstrom P, Tierney J, Wathy B, Westman J. Tetrahedron, 2001, 57: 9225. (b) Larhed
M, Hallberg A. Microwave assisted high speed chemistry; A New Technique in
Drug Discovery Today 2001, 6: 406; (c) Gedye R, Smith F, Westaway K et al. Tetrahedron
Lett., 1986, 27: 279.
[6] Vallin K S A, Emilsson P, Larhed M, Hallberg A. J. Org. Chem., 2002, 67:
6342.
[7] Leadbeated N E, Marco M. Org. Lett., 2002, 4: 2973.
[8] (a) Ueno M, Hisamoto H, Kitamori T, Kobayashi S. Chem. Commun., 2003, 936; (b)
Crosignani S, White P D, Linclau B. Organic Lett., 2002, 4: 2961.
[9] (a) Miao G, Ye P, Yu L, et al. J. Org. Chem., 2005, 70: 2332; (b) Peng Y, Song
G. Green Chem., 2003, 5: 704; (c) Dandia A, Arya K, Sati M. Synth. Commun., 2004, 34 (6):
1141; (d) Molteni V, Hamilton M M, Mao L, et al. Synthesis, 2002, (12) 1669; (e) Laskar D
D, Gohain M, Prajapati D, Sandhu J S. New J. Chem., 2002, 26: 193.
[10] (a) Jeffrey T. Tetrahedron Lett., 1994, 35: 3051; (b) Zim D,
Monteiro A L, Dupont J. Tetrahedron Lett., 2000, 41: 8199; (c) Zha Z G, Wang Y S,
Yang G, et al. Green Chem., 2002, 4: 578.
[11] Plazl I. Acta Chem. Sloy., 1994, 41: 437.
[12] (a) Dayal B, Rapole K R, Salen G, et al. Synlett., 1995, 861; (b) Shibata C, Kashima
T, Ohuchi K. Jpn. J. Appl. Phys., 1996, 35: 316.
徐小岚, 惠爱玲, 周存六, 查正根, 汪志勇
(中国科学技术大学, 合肥 230026)
摘要 首次将微波辐射应用于纯水中羰基化合物的烯丙基化反应。在Zn-SnCl2的催化作用下,微波辐射能够大幅度促进烯丙基化反应。而且,通过微波辐射实现了不活泼的烯丙基氯在纯水中的烯丙基化反应。一些传统条件下很难与烯丙基卤(溴,氯)发生反应的酮在此条件下也能够以较高产率给出相应的烯丙基化产物。与传统水溶液中的烯丙基化反应相比,不仅是反应产率有很大的提高,而且反应时间大幅度缩短,从几十个小时缩短到几分钟。
关键词 芳香醛, 水, 催化作用, 烯丙基化