http://www.chemistrymag.org/cji/2005/076044ne.htm |
Jun. 2, 2005 Vol.7 No.6 P.44 Copyright |
Zhou Jianke, Yue Qiang, Li Luhua, Li Jingxia
(Research Center of Physics and Chemistry Analysis, Hebei University; Hebei Province Key
Laboratory of Analytical Science and Technology, Baoding, 071002)
Received on Apr. 11, 2005.
Abstract Photocatalytic degradation of
diethylstilbestrol (DES) was carried out in the presence of nanosized TiO2, ZnO
and TiO2-ZnO with UV. The degradation rate was strongly influenced by the
catalyst concentration, pH value and lightsource distance. Compared with TiO2
and ZnO, DES was decomposed more efficiently using nano-TiO2-ZnO photocatalyst.
An initial DES concentration of 10mgL-1 was completely degraded with TiO2-ZnO
after 60min under the optimum conditions. The photodegradation reactions were found to
follow the first order kinetics law, and the apparent degradation rate constant was
0.0704min-1.
Keywords Diethylstilbestrol(DES), photodegradation, nano-semiconductor,
photocatalysis
Diethylstilbestrol (DES) is a estrogenic drug that is approximately 10 times as potent as estradiol-17b which is the most potent endogenous estrogen [1]. It was used rather commonly in the 1940-1960s to maintain pregnancies in USA. But after 20 years, it was discovered that women who took DES while pregnant and their daughters faced a higher risk of breast cancer, infertility, vaginal adenosis, and abnormalities of the fallopian tubes, cervix, and uterus [2-3]. In the past few years a number of papers have highlighted the potentially dangerous consequences to human and wildlife of the presence of estrogens in the aquatic environment [4-6]. In contrast to natural estrogen, estrogenic drugs such as DES are more stable and remain in the body longer than natural estrogen [7]. These estrogens are difficult to be totally removed from wastewater by the traditional treatment. So it is necessary to develop technology for its elimination and decomposition as soon as possible. Recently, there are many investigations on the degradation of estrogens in environment using heterogeneous photocatalysis technique [8-10]. But there are few articles focusing photocatalytic degradation of DES. In the present work, photodegradation of DES in aqueous suspensions is investigated using nanosized TiO2, ZnO and TiO2-ZnO as photocatalyst. The aim of this work was to investigate the different photocatalytical behaviour of these catalysts and the influence on the degradation of various parameters, such as photocatalyst concentration, pH value and lightsource distance.
1 EXPERIMENTAL
1.1 Reagents
DES (99%) was obtained from Acros Organics (Sweden). Photocatalysts were supplied by
Hebei University Research Center of Nano-Material. Methanol was HPLC grade, all other
chemicals used were of analytical grade and were used without further purification. Water
used was doubly distilled.
1.2 Photodegradation procedure
Photodegradation was conducted in a photoreactor made by ourselves. The UV lamp (25w,
254nm) was in the center of the photoreactor, keeping parallel with sample container. DES
solution of different concentration, containing precise amount of photocatalysts, was
continuously stirred with a magnetic bar. The pH of the sample solution was adjusted with
0.1N HCl or NaOH depending on the desired values. In the majority of the experiments,
temperature was kept at 25±1
1.3 Analyses
Samples were taken at given time intervals and filtered through a 0.45mm membrane filter in order to remove photocatalysts before injecting. The progress in the photodegradation of DES was followed with a HPLC (Shimadzu LC-10AT, Japan) equipped with a UV-VIS detector (Shimadzu SPD-10A, Japan) at 240nm. A separation column was a Diamonsil-C18 column (250×0.46mm, 5mm, Dikma Technologies). The mobile phase was CH3OH/H2O mixture (80%/20% v/v) at a flow rate of 1.0mlmin-1. The injection volume was 20ml. The retention time of DES was 5.6min.
2 RESULTS AND DISCUSSION
2.1 Chromatogram of DES
Nanosized TiO2 and ZnO were used commonly as photocatalyst to photodegrade
organic pollutants [11-13]. And a considerable increase of the photocatalytic
activity has been reported by combination TiO2 with ZnO [14].
Coupled semiconductor photocatalysts may increase the photocatalytic efficiency by
increasing the charge separation and extending the photo-responding range. At the same
time, their physical and optical properties are greatly modified [15]. In all
our studies, DES was degraded using TiO2, ZnO and TiO2-ZnO catalysts
to compare their photocatalytic activities. Nano-TiO2-ZnO was more efficiently,
Fig.1 is the typical chromatogram of DES in UV and UV/TiO2-ZnO system after
illumination for 30min.
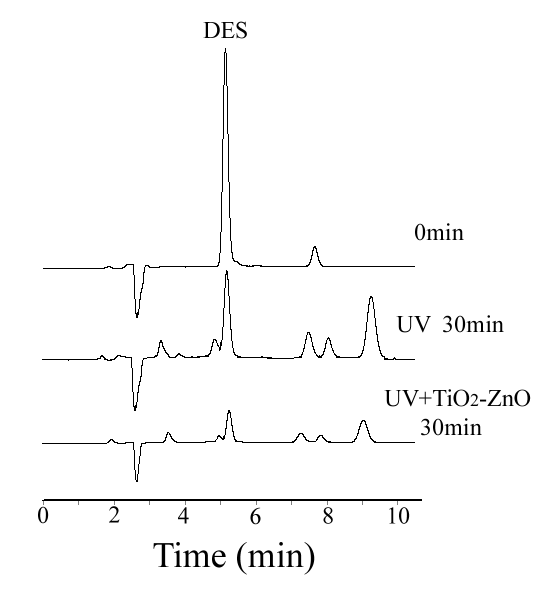
2.2 Effect of catalysts
concentration
It has been reported that the initial rate of photocatalytic degradation of many
pollutants is a function of the photocatalyst concentration [16]. The effect of
TiO2, ZnO and TiO2-ZnO concentration (0.5-3.0g/L) on the rate of photocatalytic degradation of DES was
evaluated at pH 7.0 and the results are shown in Fig. 2. These results verify that the
rate of DES degradation increases with catalysts loading up to a concentration of 2.0g/L.
The enhancement is due to the increasing volumetric photon absorption by the
photocatalyst, therefore providing a higher concentration of the charge carrier per unit
volume for DES. However, as the loading was increased beyond the optimum value, the
degradation rate decreased due to the increased opacity of the suspension and light
scattering. The penetration depth of the UV photon is lowered and less catalysts particles
could be activated. Therefore, in this study, a catalysts loading of 2.0g/L corresponding
to the highest absorption of incident photons by the photocatalyst was used for subsequent
experiments.
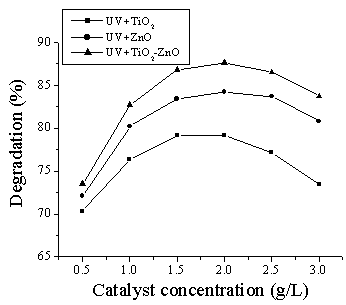
Fig. 2 Effect of catalysts concentration on the
photodegradation of DES
2.3 Effect of pH value
pH of the suspension is an important parameter in the photodegradation taking place on
semiconductor particle surfaces, since it is related to the surface charge properties of
the photocatalyst. Effect of pH on the photodegradation of DES was investigated over the
pH range of 4-10. The results are shown in Fig.3. A steady increase in the degradation of
DES is observed up to 9.0 followed by a slow decrease at higher pH value. Higher pH value
can provide higher concentration of hydroxyl ions to react with holes (h+) to
form hydroxyl radicals (OH·), subsequently enhancing the photodegradation rate of
DES. But at higher pH value, catalysts surface properties may be influenced leading to
efficiency decrease. Photocatalytic activity of ZnO is strongly influenced by pH value,
since its stability is not as good as other catalysts.
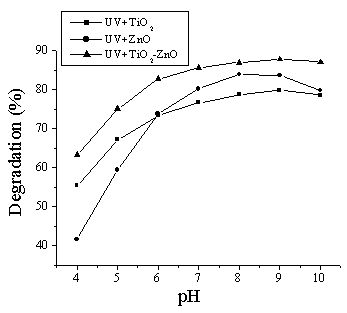
Fig. 3 Effect of pH on the photodegradation of DES
2.4 Effect of lightsource distance
The effect of lightsource distance on the photodegradation of DES was investigated by
adjusting the distance between lamp and sample container. The results are shown in Fig. 4.
The photodegradation rate of DES with TiO2-ZnO decrease sharply as increasing
the lightsource distance. Obviously, the larger lightsource distance can lead to the lower
light intensity. The influence is similar as changing lamp or wavelength [17,18].
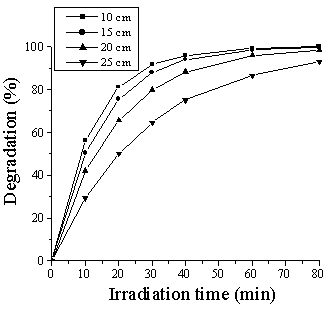
Fig. 4 Effect of lightsource distance on the photodegradation of DES
2.5 Kinetics of DES photodegradation
Many authors have reported that the kinetic behaviour of photocatalytic reaction can
be described by a modified Langmuir-Hinshelwood model [19-20].
![]()
At high substrate concentration, the adsorbed reactant molecules may
occupy all the catalytic sites on catalyst surface and this leads to zero-order kinetics.
At low concentration, the number of catalytic sites will not be a limiting factor and the
rate of degradation is proportional to the substrate concentration, in accordance with
apparent first-order kinetics. In order to confirm the speculation, ln(C0/C)
was plotted as a function of the irradiation time.
The calculated results indicated that the first-order model gives a better fit. The
apparent degradation rate constant (k) of DES under different initial concentration and
catalysts is shown in Table.1.
Table 1 The apparent degradation rate constant (k) of DES in different initial
concentration and catalyst
Catalyst |
Initial concentration ( mgL-1 ) |
Correlation coefficient r |
k (min-1) |
TiO2-ZnO |
5 |
0.992 |
0.0922 |
TiO2-ZnO |
10 |
0.998 |
0.0704 |
TiO2-ZnO |
15 |
0.993 |
0.0537 |
TiO2-ZnO |
20 |
0.997 |
0.0415 |
TiO2 |
10 |
0.994 |
0.0526 |
ZnO |
10 |
0.993 |
0.0614 |
TiO2 + hv
ZnO + hv
TiO2 (h+ + e-) + ZnO (h+ + e-)
h+ + H2O
e- + O2
2 HO2·
H2O2 + O2-
H2O2
h+ + OH-
DES +·OH
3 CONCLUSIONS
The present study clearly shows that semiconductor oxides can be used as effective
photocatalysts for degradation of DES in water. Compared with TiO2 and ZnO,
nano-TiO2-ZnO was more efficient for degradation of DES. The photodegradation
rate of DES was strongly affected by many factors, such as photocatalyst concentration, pH
value, lightsource distance. It was found that the primary photocatalytic decomposition
reaction follows a first-order kinetic law, and the apparent degradation rate constant on
TiO2, ZnO and TiO2-ZnO, k, was 0.0526, 0.0614, 0.0704min-1,
respectively.
REFERENCES
[1] Daston G P, Gooch J W, Breslin W J et al. Reproductive Toxicology, 1997, 11:
465-481.
[2] Nelson, Roxanne. Lancet Oncology, 2004, 5, 267.
[3] Zhou D N, Wu F, Deng N S. Chemosphere, 2004, 57: 283-291.
[4] Jobling S, Casey D, Rodgers G T et al. Aquatic Toxicology, 2003, 65: 205-220.
[5] Hulka B S, Moorman P G. Maturitas, 2001, 38: 103-113.
[6] Jimènez, B. Trends in Analytical Chemistry, 1997, 16 (10): 596-606.
[7] Tapiero H, Nguyen B G, Tew K D. Biomedicine and Pharmacotherapy, 2002, 56: 36-44.
[8] Watanabe N, Horikoshi S, Kawabe H et al. Chemosphere, 2003, 52: 851-859.
[9] Lee J M, Kim M S, Kim B W. Water Research, 2004, 38: 3605-3613.
[10] Kohtani S, Koshiko M, Kudo A et al. Applied Catalysis B: Environmental, 2003, 46:
573-586.
[11] Anpo M, Takeuchi M. Journal of Catalysis, 2003, 216: 505-516.
[12] Soofin C, Tsai S J, Lee Y F. Catalysis Today, 1995, 26: 87-96.
[13] Yu D Z, Cai R X, Liu Z H. Spectrochimica Acta Part A, 2004, 60: 1617-1624.
[14] Ding S W, Wang L Y, Zhang S Y et al. Chinese Journal of Inorganic Chemistry (Wuji
Huaxue Xuebao), 2003,19 (6): 631-635.
[15] Li X Z, Li F B, Yang C L et al. Journal of Photochemistry and Photobiology A:
Chemistry, 2001, 141: 209-217.
[16] Stafford U, Gray K A, Kamat P V. Journal of Catalysis, 1997, 167: 25-32.
[17] Tommasini S, Calabrò M L, Donato P et al. Journal of Pharmaceutical and
Biomedical Analysis, 2004, 35: 389-397.
[18] Nagai Y, Nakamura D, Ueno H et al. Polymer Degradation and Stability, 2005,88:
256-260.
[19] Zhang F L, Zhao J C, Shen T et al. Applied Catalysis B: Environmental, 1998, 15:
147-156.
[20] Wei H B. Shanghai Environmental Sciences (Shanghai Huanjing Kexue), 1995, 14 (3):
7-10.
周建科 岳强 李路华 李敬霞
(河北大学理化分析中心,河北省分析科学技术重点实验室,保定 071002)
摘要 考察了己烯雌酚在纳米TiO2、ZnO和TiO2-ZnO催化作用下紫外光降解情况。降解速率受催化剂用量、pH值和光源距离的影响较大。与TiO2和ZnO相比,纳米TiO2-ZnO可以更有效的降解己烯雌酚。在TiO2-ZnO催化的最佳条件下,初始浓度为10mg/L的己烯雌酚光照60min即降解完全,光降解反应遵从动力学一级反应,表观降解速率常数为0.0704min-1。
关键词 己烯雌酚,光降解,纳米半导体,光催化