http://www.chemistrymag.org/cji/2005/076046ne.htm |
Jun.6, 2005 Vol.7 No.6 P.46 Copyright |
A convenient method for the synthesis of the two compounds of sex pheromones of Hyphantria Cunry
Yan Zankai1, Zhang Zhongning2(1Department of Chemistry and Environmental Engineering, Yangtze University, Jingzhou, 434025; 2Institute of Zoology, Chinese Academy of Sciences, Beijing 100080, China)
The fall webworm moth, Hyphantria Cunea (Drury), is
one of the most important pest of fruit, nut, and shade tree in China.[1] The
sex pheromones of Hyphantria Cunea related to the mating behavior has been
intensively studied in the field, and could be used for monitoring and controlling.[2]
Hence the synthesis of sex pheromones of Hyphantria Cunea will play an important
role in the protecting of forest.
Since the two components, (9z, 12z)-octadecadienal and (9z, 12z,
15z)-octadecatrienal, have been identified as major components of the sex pheromones of Hyphantria
Cunea by Hill,[3] the two unsaturated aliphatic aldehydes had been
synthesized by Elysian and Naomi.[4] The method for the preparation of the two
aldehydes was that linoleyl and linolenyl alcohols were obtained by direct reduction of
ethyl linoleic and linolenic acids with lithium aluminium hydride and then were converted
into the aldehydes by oxidation with pyridinium chlorochromate. However, the oxidation
reaction was the key step and found to be very sluggish, and a large excess of reagent was
repaired to obtain reasonable yield of aldehyde.
In order to found out an economical way of preparation of the two
aldehydes, our synthetic routes are show in Scheme 1. The two aldehydes were prepared from
linoleic and linolenic acids, which were reduced with lithium aluminium hydride and then
the oxidation of the corresponding alcohols with poly (vinylpyridinium dichromate),
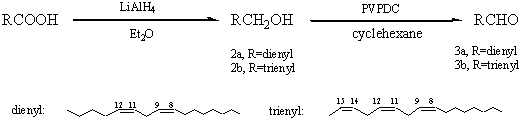
Scheme 1 Linoleic and linolenic acids, which are widely distributed in nature, could obtained easily. Treatmeat of compound ( 2 ) with PVPDC gave the target compound ( 3 ) in good yield, the final product could be separated by simple filtration. Furthermore, the polymeric reagent should be easy to prepare, have a capacity sufficient for use on a practical scale, and be designed in such a way that the spent reagent can be regenerated to its initial activity in an easy fashion[5].
The general procedure is as follows: both of the alcohols were prepared by the reported procedure[6]. The PVPDC was soaked with water for 10 minutes, and then was filtered. To a mixture of the alcohols ( 2 ) (2.0 g) in cyclohexane (20.0 mL) was added with PVPDC (10 g) at 70ºC and the mixture was stirred at room temperature for 24 h. The polymeric reagent was filtered and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluted with ethyl acetate-petroleum ether = 1:10) to gave the aldehydes ( 3 ) in over 92-94 % yield.
Both of the aldehydes were identified as unsaturated fatty aldehydes with the same number of carbon atom as relative acids, and the same mass of molecule of aldehydes as quantity of that calculated by 1H NMR, GC-MS. No peak approach at ca 970 cm-1 in IR, which indicated that the double bond present are of the Z configuration. The spectral data[7] obtained for two target compounds were identical with those in the literature [3, 4].
This method was simple and convenient for the preparation of (9z, 12z)-octadecadienal and (9z,12z,15z)-octadecatrienal, compared to the other methods available in the literature.
Acknowledgements The authors thank express to the National Natural Sciences Foundation of China for financial support.
REFERENCES AND NOTES[1] Zhang S F, Yu C Y. Hyphantria Cunea., Science press, Beijing (in Chinese), 1985, 138-140.
[2] Zhang Q H, Schlyter F J. Appl. Ent., 1996, 120, 467-476.
[3] Hill A S, Kovalav B G, Nikolaeva L N. J. Chem. Ecol., 1982, 8, 383-396.
[4] (a) Eglinton G, Hunneman D H, McCormick A. Org. Mass. Spectrum., 1968, 1, 593-611.
(b) Naomi C D, Gerhard G, Reging G. J. Chem. Ecol., 1999, 25 (10), 2229-2245.
[5] Jean M J. J. Org. Chem., 1978, 43 (13), 2618-2621.
[6] Ligthelm S P, Rudloff E V, Sutton D A. J. Am. Chem. Soc., 1950, 3187-3190.
[7] (a) 3a: bp 142-143 ºC/1333Pa. IR, n: 1729 (C=O), 3008, 2926, 2855, 1464, 723. 1H NMR, d: 0.91(t, 3H, -CH3), 1.29 (s, 8H, 4-6, 16, -CH2), 1.34 (s, 6H, 7, 15, 17, -CH2), 1.65 (m, 2H, -CH2), 2.06 (m, 4H, 8, 14, -CH2), 2.45 (m, 2H, 2, -CH2), 2.79 (t, 2H, 11, -CH2), 5.39 (m, 4H, 9, 12, -CHCH), 9.79(s, 1H, -CHO). MS, m/z: 264 (M+), 95 (27), 67 (82), 55 (62), 41(100).
(b) 3b: bp 143-145ºC/1333Pa. IR, n: 1727 (C=O), 3011, 2928, 2855, 1463, 721. 1H NMR, d:1.00 (t, 3H, -CH3), 1.26 (s, 6H, 4-6, -CH2), 1.32(s, 2H, 7, -CH2), 1.66 (m, 2H, 3, -CH2), 2.08 (m, 4H, 8, 17, -CH2), 2.45 (m, 2H, 2, -CH2), 2.81(t, 4H, 11,14, -CH2), 5.36(m, 6H, 9, 12, 15, -CHCH), 9.77 (s, 1H, -CHO). Ms, m/z: 262 (M+), 108 (18), 93 (29), 79 (81), 67 (60), 55(43), 41(100).
美国白蛾性信息素两个成分的一种简便合成方法
严赞开1, 张钟宁2
(1长江大学化学与环境工程学院,荆州 434025;2中国科学院动物研究所,北京 100080)
摘要 以亚油酸和亚麻酸为原料,制备了美国白蛾性信息素的两个主要成分, (9z, 12z)-十八碳二烯醛和 (9z, 12z, 15z)-十八碳三烯醛,所合成的化合物均进行了IR、1H NMR及GC-MS波谱表征。用聚乙烯二铬酸吡啶氧化醇成醛,氧化剂不仅有很高的氧化效率且易于再生;另外,氧化剂不溶于溶液,只需简单的过滤就可将产物分离纯化。该方法原料易得,操作简便,合成效率高。
关键词 性信息素,美国白蛾,聚乙烯二铬酸吡啶